
Volume 3, Issue 4, 2025
Editorial
Reframing the Approach to Predatory Journals; Embracing a 'Non-Recommended Journal' Model
Abdullah Khalid Omer
For more than a decade, the academic publishing community has been locked in a battle against “predatory journals.” These are commonly understood as outlets that exploit the open access model by charging fees to authors without providing genuine peer review or editorial services [1]. While this campaign has been well-intentioned, its implementation has been riddled with inconsistencies and collateral damage. It is time to re-evaluate our approach—and a promising alternative has recently been proposed.
At the 18th Meeting of the European Association of Science Editors (EASE), Kakamad et al. introduced the concept of the Non-Recommended Journal (NRJ), offering a more nuanced and constructive way to classify questionable journals. Their proposal, outlined in a poster presented at the event, acknowledges a critical truth that the current binary model overlooks: not all low-quality or problematic journals are predatory, and not all accused journals are guilty [2].
One of the core issues with the term “predatory” is its lack of a universally accepted definition. Attempts to label journals as predatory can often be subjective and based on flawed or incomplete criteria. This ambiguity has led to wrongful accusations and the potential defamation of emerging or under-resourced journals that are making genuine efforts to improve. Worse still, some well-established journals exhibit questionable practices yet avoid scrutiny simply because they don’t fit the “predatory” mold [3].
The NRJ framework reframes the discussion by focusing not on intention, but on recommendation. Rather than asking whether a journal is maliciously exploitative, the NRJ model asks whether a journal meets acceptable standards of transparency, editorial rigor, and academic integrity. Journals that do not meet these standards—whether due to deliberate misconduct or lack of infrastructure can be flagged as “non-recommended” without implying criminality or predation [2].
This shift in terminology allows for a more flexible and inclusive way to monitor journal quality. It accounts for the so-called “borderline journals,” which may not be outright deceptive but still fail to uphold scholarly standards. By avoiding the inflammatory label of “predatory,” the NRJ system reduces the risk of reputational harm while still guiding authors, reviewers, and institutions toward better publishing decisions.
Moreover, the NRJ approach invites continuous re-evaluation. Journals can move in and out of this category based on demonstrated improvements, providing a growth mindset rather than cementing stigmas. This dynamic classification also encourages more transparent criteria, ideally informed by independent watchdogs or academic associations rather than commercial blacklists.
It is time we recognize the complexity of the academic publishing ecosystem and evolve beyond the simplistic predator-prey narrative. The NRJ concept represents a practical, fair, and forward-thinking step in that direction. As the academic world continues to grapple with questions of quality, ethics, and accessibility, such innovations are not just welcome, they are essential.
Original Articles
Predictors of False-Negative Axillary FNA Among Breast Cancer Patients: A Cross-Sectional Study
Lana R. A. Pshtiwan, Sakar O. Arif, Harzal Hiwa Fatih, Masty K. Ahmed, Shaban Latif, Meer M....
Introduction
Fine-needle aspiration (FNA) is commonly used to investigate lymphadenopathy of suspected metastatic origin. The current study aims to find the association between nodal characteristics and cancer-related factors with the rate of false-negative preoperative FNA.
Methods
This retrospective, single-center, cross-sectional study included breast cancer patients with negative preoperative axillary FNA results who underwent postoperative histopathological evaluation. Data were collected from electronic medical records, including clinical, imaging, cytological, and pathological findings. Patients with incomplete records, non-axillary or inconclusive FNAs, positive preoperative FNAs, or unsampled axillae postoperatively were excluded. Key variables analyzed included lymph node size, cortical thickness, tumor grade, histological type, immunohistochemical subtype, and metastatic patterns.
Results
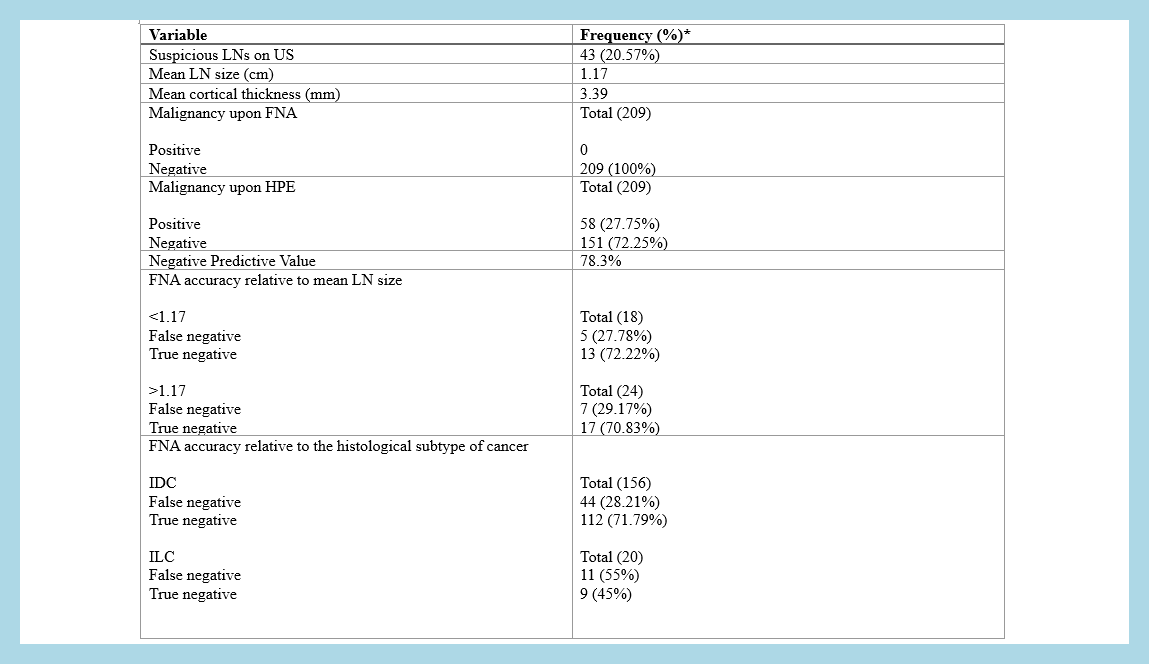
A total of 209 negative axillary FNA samples were analyzed, with a mean patient age of 46.13 years. Invasive ductal carcinoma was the most common diagnosis, and ER-positive tumors were the predominant subtype. Ultrasonography identified suspicious axillary nodes in 20.57% of cases. Histopathology revealed a 27.75% false-negative rate, with a negative predictive value of 78.3%. Larger lymph node size and cortical thickness exhibited lower false-negative rates, while histologic type and ER status showed significant associations with false-negative outcomes (P < 0.05).
Conclusion
The 27.75% false-negative rate of preoperative FNA remains concerning and may not be sufficiently low to justify foregoing definitive axillary staging. The current study found significant associations between false-negative FNA rates and histological subtype and ER status, the latter of which is not explicitly mentioned in the literature.
Echinococcus granulosus in Environmental Samples: A Cross-Sectional Molecular Study
Thamr O. Mohammed, Shvan L. Ezzat, Hawnaz S. Abdullah, Sangar J. Qadir, Aga K. Hamad, Sahar A....
Introduction
Echinococcosis, caused by tapeworms of the Echinococcus genus, remains a significant zoonotic disease globally. The disease is particularly prevalent in areas with extensive livestock farming. Humans primarily acquire infection through consumption of contaminated food or water, often from environmental contamination by definitive host feces. This study aimed to detect the presence of E. granulosus DNA (deoxyribonucleic acid) in water and vegetable samples collected from Sulaymaniyah Governorate, Iraq.
Methods
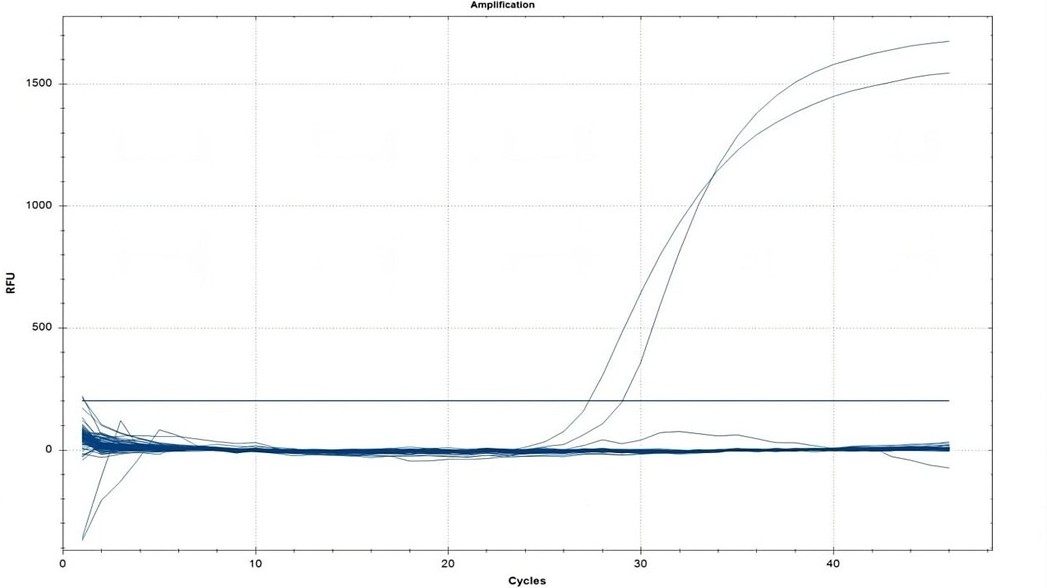
A cross-sectional study was conducted in Sulaymaniyah Governorate, Iraq, in April 2025. Water and vegetable samples were collected from both urban and rural areas. DNA extraction was performed from all samples, and E. granulosus DNA was explored using a qPCR (quantitative polymerase chain reaction) assay. Sample processing included filtering water, washing vegetables, and DNA extraction under optimized conditions.
Results
A total of 245 samples, comprising 98 (40.0%) water samples and 147 (60.0%) vegetable samples, were analyzed, with 111 (45.3%) from urban and 134 (54.7%) from rural areas. Despite the comprehensive sampling, no E. granulosus DNA was detected in any sample. All control reactions yielded positive results, but no amplification was observed in the field samples, indicating the absence of E. granulosus contamination.
Conclusion
This study found no evidence of E. granulosus DNA in water or vegetable samples from Sulaymaniyah, Iraq, suggesting a low likelihood of environmental contamination in this region. but seasonal changes, the restricted sample size, and methodological limitations mean that the presence of contamination cannot be completely excluded.
Carcinoma ex Pleomorphic Adenoma: A Case Series and Literature Review
Abdulwahid M. Salih, Rebaz M. Ali, Ari M. Abdullah, Aras J. Qaradakhy, Ahmed H. Ahmed, Imad J....
Introduction
Carcinoma ex pleomorphic adenoma (CXPA) is a rare malignant salivary gland tumor that can lead to severe complications and carries a risk of distant metastasis. This study aims to provide a comprehensive overview of CXPA through a case series and a review of the literature.
Methods
This was a single-center retrospective case series. The patients were included from November 2018 to December 2024. All confirmed cases of CXPA that were diagnosed and managed with complete clinical data were included in this study. Cases with incomplete data were excluded.
Results
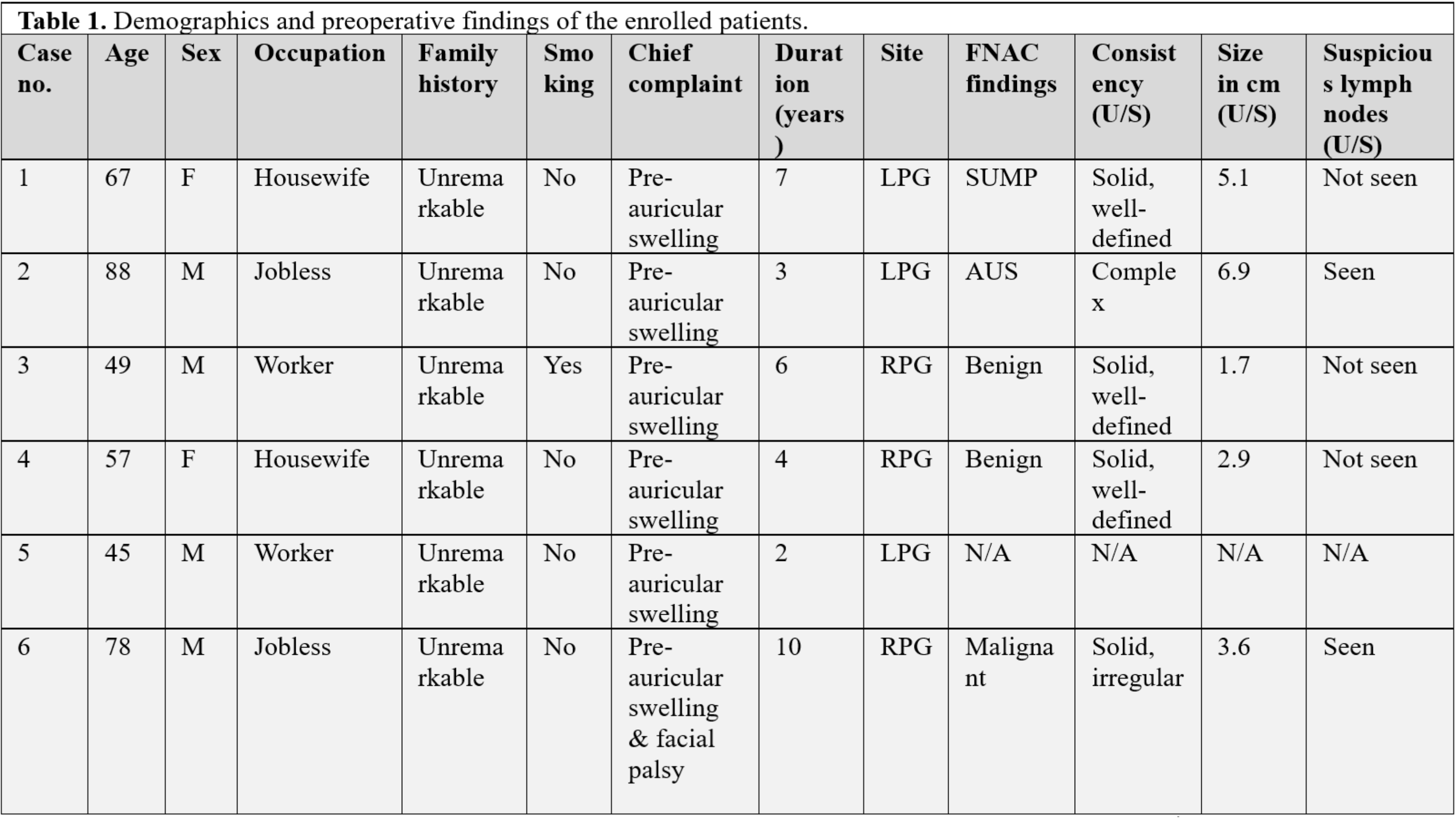
Six patients were included, with ages ranging from 45 to 88 years (mean ± SD: 64 ± 15.36; median: 62). Most were male (66.7%), with an even distribution of occupations. All presented with preauricular swelling lasting 2 to 10 years, and three had left-sided tumors. Fine needle aspiration identified 33.3% as benign and 16.7% as malignant. Ultrasound examination showed solid tumors in four cases, three of which were well-defined. Three (50%) underwent total parotidectomy, and three (50%) underwent superficial parotidectomy. Histopathological examination revealed adenocarcinoma ex pleomorphic adenoma in 50% and squamous cell carcinoma ex pleomorphic adenoma in 16.7%. Tumor sizes ranged from 3.5 to 6 cm (mean: 4.73 ± 1.24 cm). Capsular invasion was present in all cases, with lymph node involvement in 33.3%, lympho-vascular invasion in 16.7%, and perineural invasion in 50%. Adjuvant therapy included radiotherapy or chemoradiotherapy.
Conclusion
Although CXPA is very rare, it is a serious condition; surgical approach with or without adjuvant therapy may result in preferable outcomes.
Perceptions of Telemedicine and Rural Healthcare Access in a Developing Country: A Case Study of Bayelsa State, Nigeria
Ebidor Lawani-Luwaji, Chibuike Frederick Okafor
Introduction
Telemedicine is the remote delivery of healthcare services using information and communication technologies and has gained global recognition as a solution to address healthcare disparities. This study explores the perceptions of telemedicine and its potential to improve rural healthcare access in a developing country through the insights of undergraduate Medical Laboratory Science students.
Methods
A descriptive cross-sectional study was conducted among 42 fourth-year students of the Medical Laboratory Science program. Respondents completed a structured questionnaire that assessed their awareness, familiarity, perceived benefits and barriers to telemedicine, and their views on its applicability in rural Bayelsa.
Results
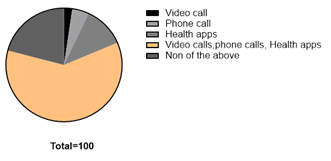
The findings indicated that while the majority of respondents (60.5%) were aware of telemedicine, their understanding of specific types, such as asynchronous and synchronous telehealth, was limited. The main perceived benefits were improved healthcare access (48.8%) and reduced costs (18.6%). Acceptance levels varied, with 47.6% endorsing telemedicine, while others remained uncertain or sceptical.
Conclusion
The study reveals enthusiasm and knowledge gaps among future healthcare professionals regarding telemedicine. It highlights the need for targeted education, digital literacy, and infrastructure investment to enable telemedicine in rural Nigerian communities.
Review Articles
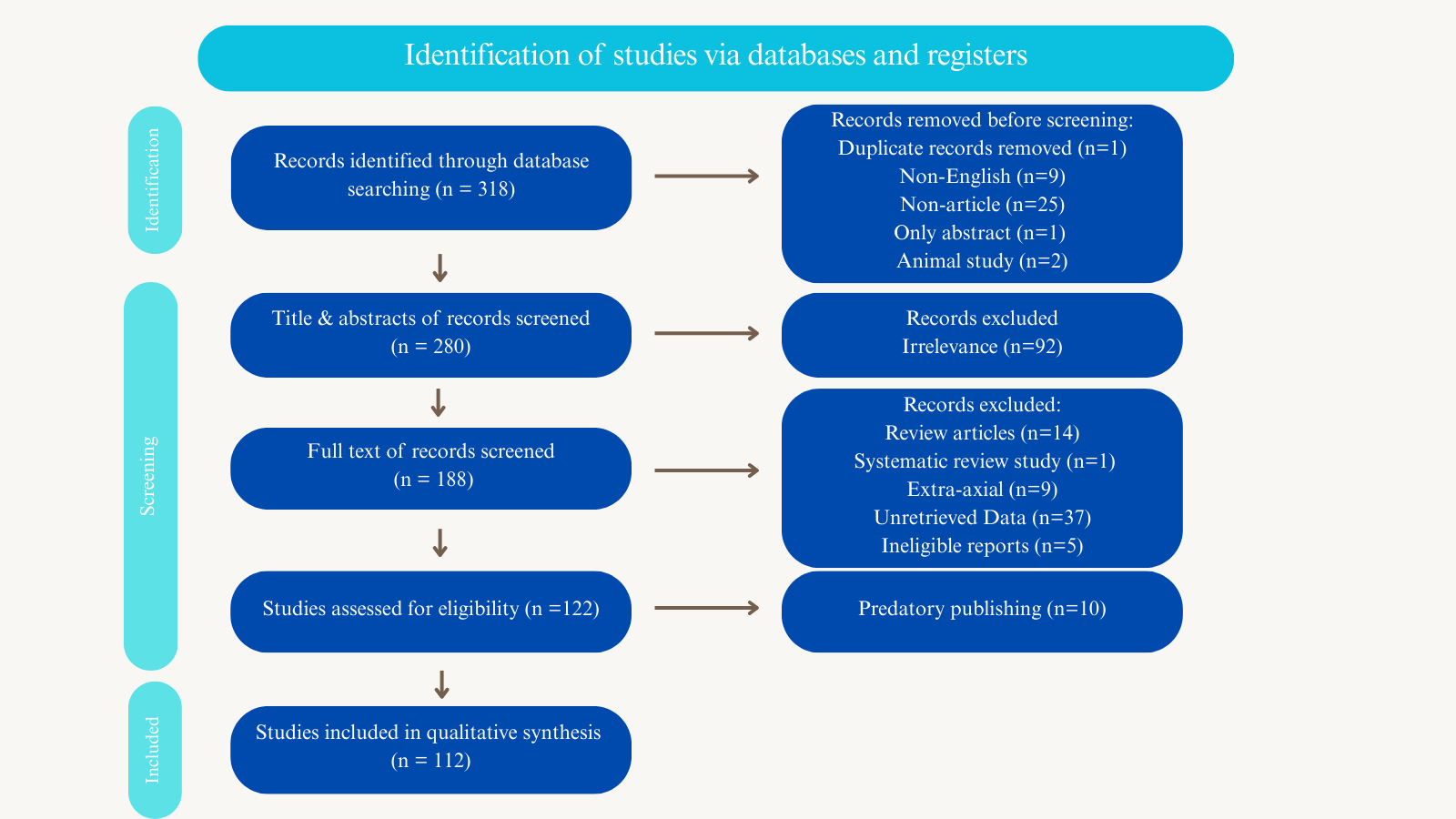
Hydatid Disease of The Brain Parenchyma: A Systematic Review
Fattah H. Fattah, Azad Star Hattam, Zana Omar kak Abdullah, Khanda A. Anwar, Rezhen J. Rashid,...
Introduction
Isolated brain hydatid disease (BHD) is an extremely rare form of echinococcosis. A prompt and timely diagnosis is a crucial step in disease management. This study is a systematic review of studies on intra-parenchymal BHD.
Methods
Studies that had the following properties were included: 1) The intra-parenchymal brain infection had been confirmed by diagnostic modalities, surgical findings, or histopathology. 2) The patient details were provided in the study. 3) The cystic lesion [s] were located intracranially.
Results
Altogether, 112 studies with a sample size of 178 cases met the inclusion criteria. Males (60.1%) showed a higher prevalence of the disease than females (38.2%). Most of the cases (64%) were affected during the first and second decades of their lives. Left-side multi-lobe involvement was the most common type of involvement (28.1%), followed by right-side multi-lobe involvement (26.4%). Surgery was the primary treatment option (97.2%), with the Dowling technique or the modified Arana-Iniguez method as the preferred approach. The total recurrence and mortality rates were 7.3% and 3.4%, respectively.
Conclusion
The definitive treatment for BHD is surgery, with the aim of removing cysts intact or excising mass lesions completely. A history of cyst rupture during operation may increase the likelihood of recurrence, and an extensive follow-up is required.
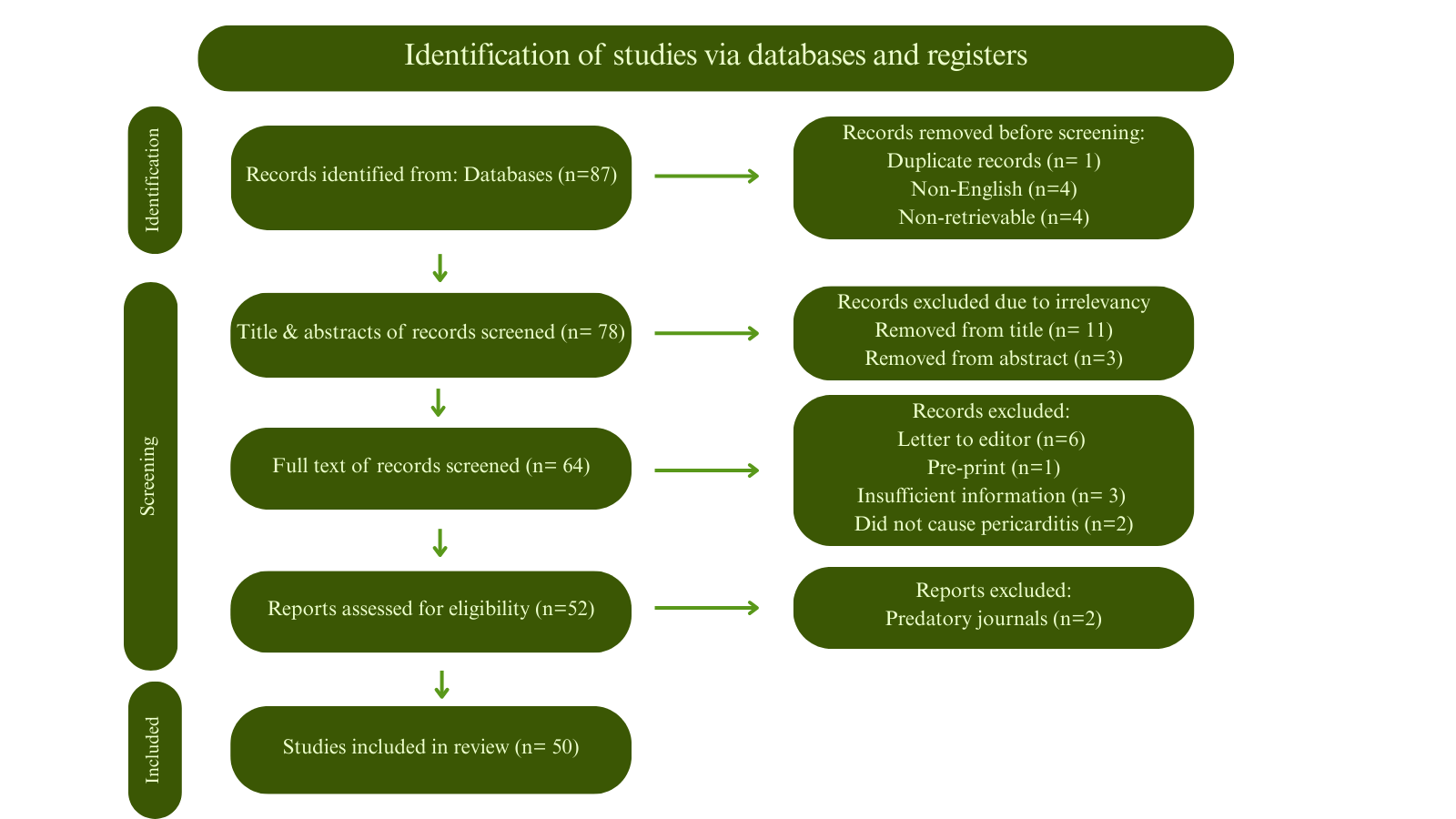
Emerging Evidence of IgG4-Related Disease in Pericarditis: A Systematic Review
Dilan Hikmat, Saman Al Barznji, Adolfo Martinez, Akhil Gaderaju, Mohammed Alaa Raslan, Mohammad...
Introduction
Immunoglobulin G4-related disease (IgG4-RD) is a recently identified immune-mediated condition that is debilitating and often overlooked. While IgG4-RD has been reported in several organs, this study reviews cases where IgG4-RD caused pericarditis.
Methods
A systematic search was conducted from inception until March 1, 2025. All age groups and both sexes with confirmed pericarditis were included, along with the following inclusion criteria: 1) Patients with pericardial biopsy showing IgG4/IgG ratio of >40%. 2) Patients with pericardial biopsy revealing IgG4/HPF of >10. 3) Patients who had confirmed IgG4-RD from other organ biopsies through IgG4 staining, or diagnostic imaging suggestive of IgG4-RD, or pericardial biopsy with classic IgG4-RD histopathologic patterns, with elevated serum IgG4 levels, provided no other diagnosis was more likely.
Results
A total of 50 patients were included, with a mean age of 64.86±15.79 years. There were 36 (72%) males. The most common presenting symptom was dyspnea in 27 (54%) patients. Different pericardial involvements were reported, including pericardial thickening 37 (74%), constrictive pericarditis 28 (56%), pericardial effusion 23 (46%), pericardial calcification 6 (12%), and pericardial nodule 5 (10%). In 28 (56%) patients, only the pericardium was affected. In addition to the pericardium, eight (16%) patients had one other organ affected, and 11 (22%) patients had two additional organs affected. Two (4.5%) cases ended in demise.
Conclusion
Although rare, IgG4-RD can cause pericarditis, leading to pericardial thickening, effusion, constrictive pericarditis, or the formation of pericardial nodules. Treatment with corticosteroids or pericardiectomy has been associated with favorable outcomes.
Case Reports
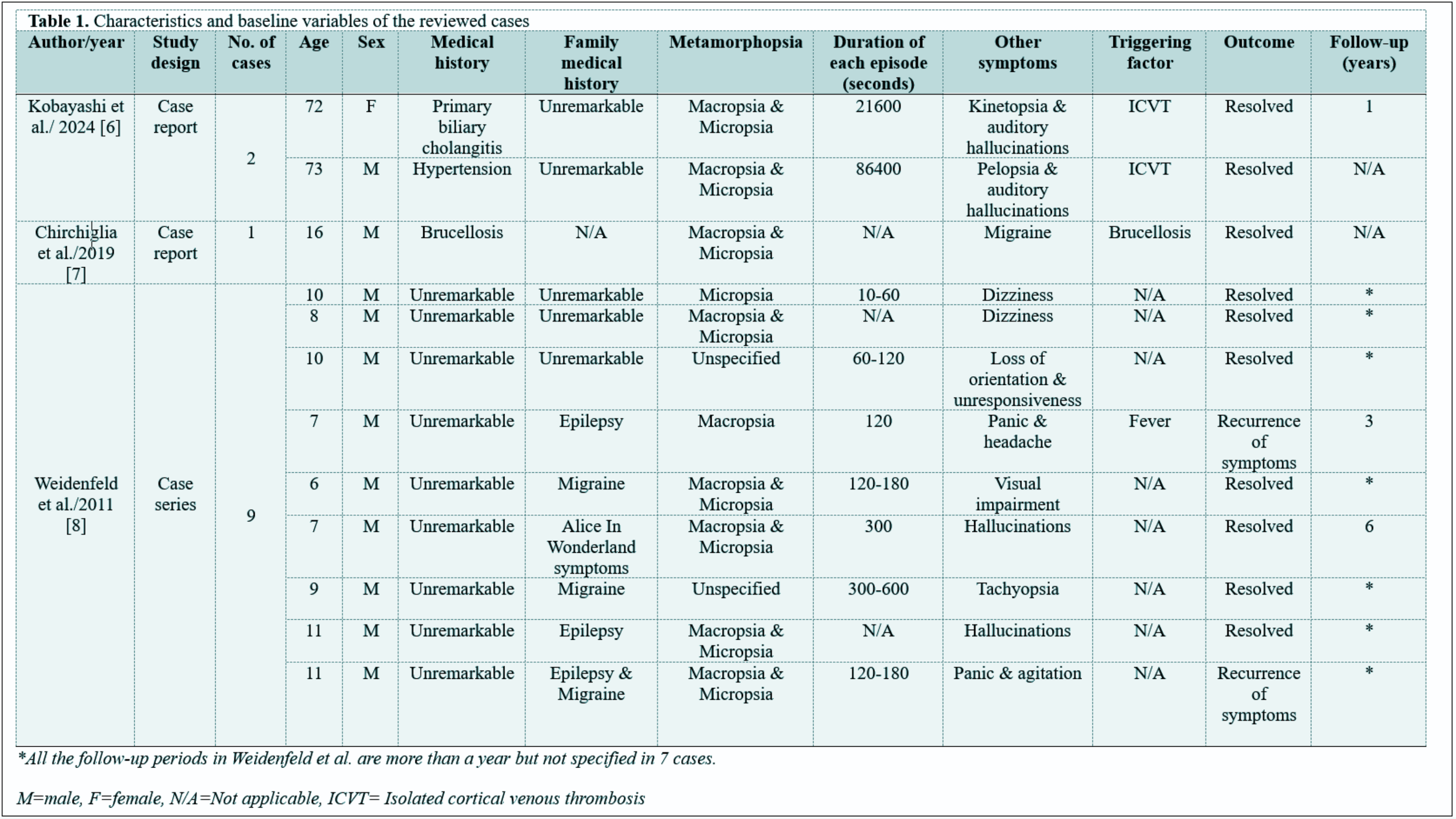
Unusual Presentation of Alice in Wonderland Syndrome: A Case Report and Literature Review
Yadgar N. Abbas, Meer M. Abdulkarim, Mohammed Q. Mustafa, Diyar A. Omar, Kayhan A. Najar, Karokh...
Introduction
Alice in Wonderland Syndrome (AIWS) is an uncommon and frequently overlooked neuropsychiatric condition, marked by brief episodes of altered visual and somatosensory perception. This report presents a case of AIWS, highlighting the disorder's unusual nature.
Case presentation
A 21-year-old female sought evaluation due to episodic visual distortions and altered body perceptions lasting for six months, often accompanied by migraines. These episodes, including micropsia, macropsia, and derealization, typically occurred multiple times a week and lasted several minutes, with no clear triggers but worsening with stress or irregular sleep. She had a history of similar, less intense episodes in childhood. Neurological examination, MRI, EEG, and blood tests were all normal. She was diagnosed with AIWS related to her migraines and was prescribed propranolol, a stress management strategy, regular sleep, and cognitive behavioral therapy.
Literature review
Sixteen cases of AWIS were reviewed, of which only one had a family history of the condition. All of them experienced perceptual distortions, with macropsia and micropsia together appearing in eight cases, and hallucinations were present in four. Duration of symptoms ranged from one minute to one day. The triggering factors included Isolated cortical venous thrombosis and brucellosis. Treatments included lacosamide and paroxetine. Recurrence of symptoms was recorded in two patients.
Conclusion
Alice in Wonderland Syndrome is mostly a benign condition that can be resolved spontaneously or treated according to its associated cause; propranolol, improved sleep schedule, and cognitive behavioral therapy might yield good outcomes.
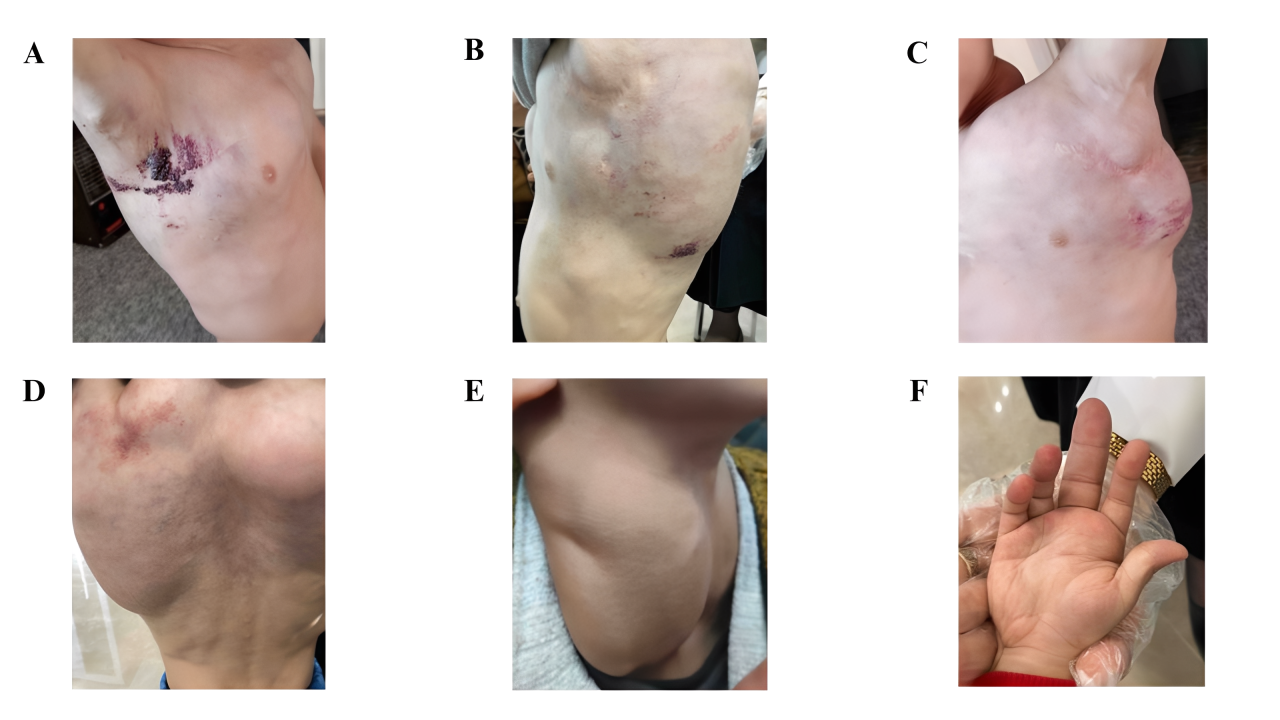
Unusual Presentation of Mixed Lymphatic Malformation: A Case Report with Literature Review
Ronak S. Ahmed, Shvan O. Siddiq, Lawen J. Mustafa, Omed M. Hussein, Sakar O. Arif, Dana HB....
Introduction
There is a scarcity of reports on mixed lymphatic malformation. This case highlights a child with an extensive mixed lymphatic malformation, disfiguring multiple parts of the body.
Case presentation
A 3.5-year-old boy presented with multiple vesicular “frog-spawn” lesions that affected the right mid-axillary region, left axilla, and left upper back. The patient also had marked bilateral cervical swelling, extending laterally on both sides, with prominent bulges measuring approximately up to 10 cm that extended to involve the entire right upper limb dawn to the hand. Bilateral axillary swelling was observed. Additionally, swelling was evident in the entire middle and upper back, with a pronounced rounded bulge in the right upper back. Surgical intervention of the left axillary mass was performed, which was consistent with macrocystis lymphatic malformation on histopathology, but the condition recurred during follow-up.
Literature review
Few cases of microcystic lymphatic malformation with extensive deep lymphatic involvement have been reported in the literature. There was a female predilection with reported involvement of the forearm, thigh, vulva, axilla, jaw, and even the tongue. The patients' ages ranged from infancy to adulthood, with some patients complaining of skin lesions and recurrent cellulitis for years, sometimes leading to sepsis and death. There is variable outcome for surgical intervention in such cases.
Conclusion
Mixed lymphatic malformation can present extensively affecting several parts of the body causing significant disfigurement with possible recurrence after surgical intervention.
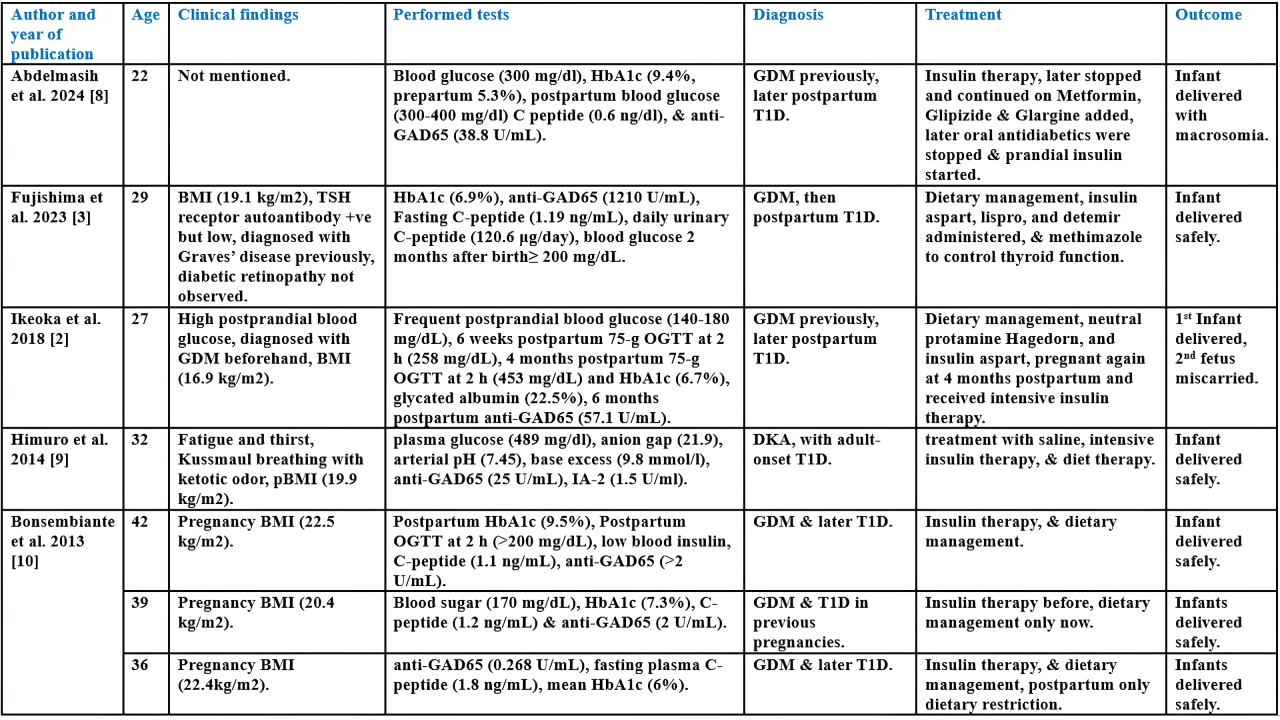
Pregnancy and Challenging Transient Anti-GAD65 Positivity: A Case Report with Literature Review
Shaho F. Ahmed, Sharaza Q. Omer, Rawezh Q. Salih, Huda M. Muhammad, Nahida H.A Ahmed, Jamal M....
Introduction
During pregnancy, women may develop blood glucose abnormalities like gestational diabetes mellitus (GDM) or, rarely, type 1 diabetes (T1D), which can lead to complications. Anti-GAD65 is a key antibody used in diagnosing T1D. This study presents a rare case of T1D developing a week before birth, with transient anti-GAD65 positivity.
Case presentation
A 38-year-old patient who delivered a baby a week earlier and had high blood glucose was admitted to the hospital with shortness of breath, chest tightness, abdominal pain, generalized weakness, nausea, and repeated vomiting. She had elevated anti-GAD65 and was diagnosed with T1D and diabetic ketoacidosis. Insulin injection was prescribed, with lifestyle modifications, later oral hypoglycemic medications added. After a few months, both anti-IA2 and anti-GAD65 antibodies were negative.
Literature review
Seven cases of T1D during or after pregnancy were reviewed. Six reported BMI. The mean HbA1c was >7.63%. Mean anti-GAD65 was 190.74 U/mL, two were borderline and one negative. Six had previously diagnosed GDM. Treatments varied, including insulin and dietary management. All infants were safely delivered, one miscarried in a subsequent pregnancy. Insulin resistance increases during pregnancy due to hormonal changes, raising the risk of GDM, T1D, and type 2 diabetes. Emerging postpartum, often indicated by anti-GAD65 antibodies, though levels can fluctuate. Cases show complications like preeclampsia, DKA, and miscarriage. Early detection, strict glucose control, and monitoring antibody patterns are critical for managing risks and improving maternal and fetal outcomes.
Conclusion
Blood glucose should be monitored during pregnancy, and anti-GAD65 may signal T1D, requiring appropriate management.
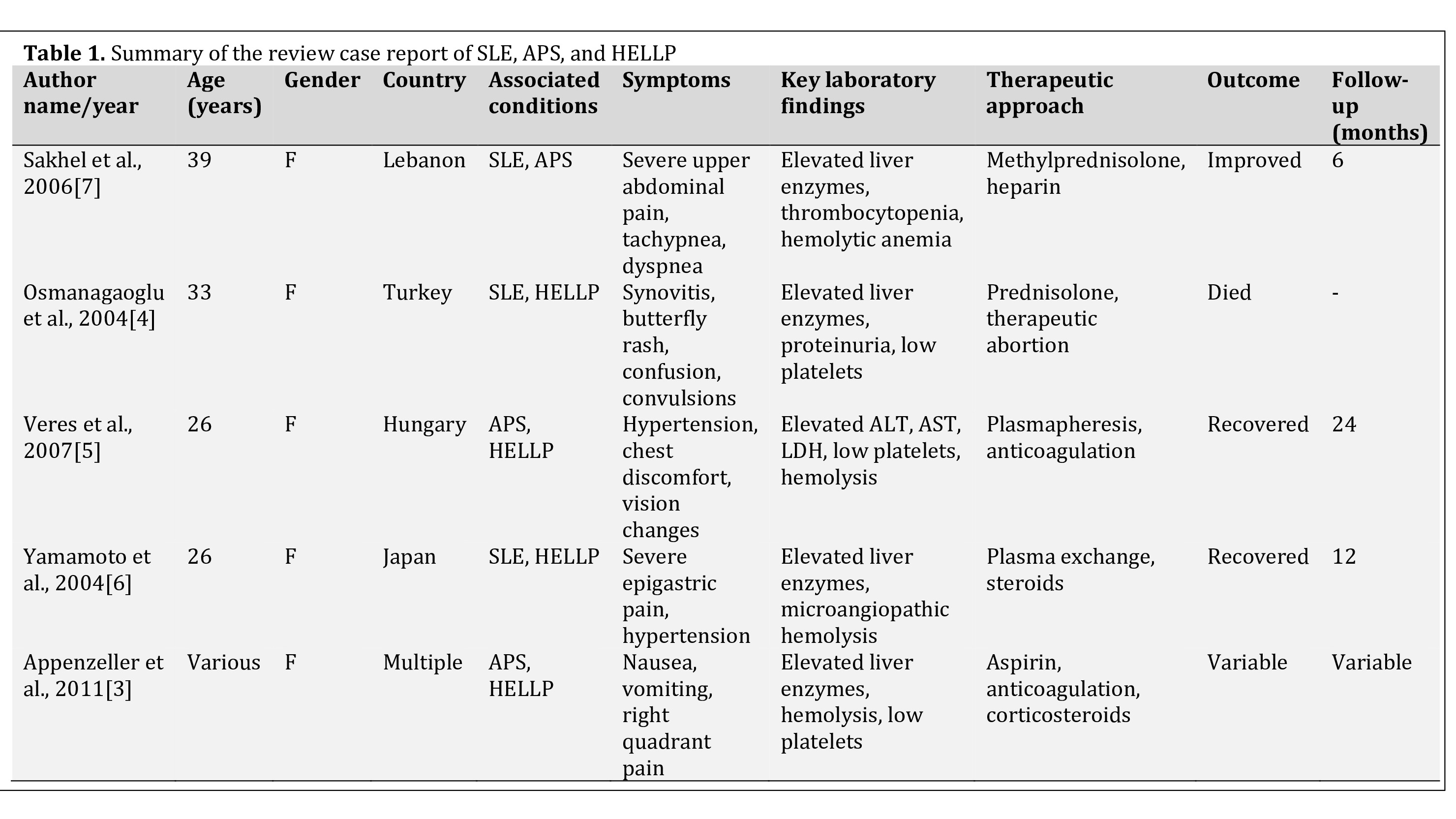
Three in One: Systemic Lupus Erythematosus, HELLP Syndrome, and Antiphospholipid Syndrome: A Case Report and Literature Review
Sivan H. Salih, Dana T. Gharib, Nahida H. Ahmad, Hoshmand R. Asaad, Ronak S. Ahmed, Huda M....
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease commonly affecting women of reproductive age. Its overlap with HELLP syndrome (Hemolysis, Elevated Liver Enzymes, and Low Platelet Count) and antiphospholipid syndrome (APS) during pregnancy is rare and presents diagnostic and therapeutic challenges due to shared clinical and laboratory features. This report describes a case of pre-existing SLE complicated by the concurrent occurrence of SLE flare, HELLP syndrome, and APS during pregnancy.
Case presentation
A 26-year-old female with known SLE presented with right upper quadrant abdominal pain and hypertension two days following a mid-trimester abortion at 22 weeks of gestation. Laboratory evaluation revealed thrombocytopenia, elevated liver enzymes, and hemolysis, consistent with HELLP syndrome. The presence of lupus anticoagulant and anticardiolipin antibodies, in conjunction with her obstetric history, supported the diagnosis of APS. The patient was treated with high-dose corticosteroids and anticoagulation, resulting in significant clinical improvement.
Literature review
A review of literature highlights the consistent presentation of nonspecific symptoms such as abdominal pain, nausea, and hypertension in patients with overlapping SLE, HELLP syndrome, and APS. Laboratory findings often reveal thrombocytopenia, elevated liver enzymes, and hemolysis, reflective of underlying microangiopathic processes. Therapeutic strategies typically involve corticosteroids and anticoagulation, with plasmapheresis reserved for severe cases.
Conclusion
The coexistence of SLE flare, HELLP syndrome, and APS during pregnancy is a rare and complex condition that requires careful evaluation. Early recognition and appropriate management are crucial for achieving favorable outcomes.
