Unusual Presentation of Mixed Lymphatic Malformation: A Case Report with Literature Review
Abstract
Introduction
There is a scarcity of reports on mixed lymphatic malformation. This case highlights a child with an extensive mixed lymphatic malformation, disfiguring multiple parts of the body.
Case presentation
A 3.5-year-old boy presented with multiple vesicular “frog-spawn” lesions that affected the right mid-axillary region, left axilla, and left upper back. The patient also had marked bilateral cervical swelling, extending laterally on both sides, with prominent bulges measuring approximately up to 10 cm that extended to involve the entire right upper limb dawn to the hand. Bilateral axillary swelling was observed. Additionally, swelling was evident in the entire middle and upper back, with a pronounced rounded bulge in the right upper back. Surgical intervention of the left axillary mass was performed, which was consistent with macrocystis lymphatic malformation on histopathology, but the condition recurred during follow-up.
Literature review
Few cases of microcystic lymphatic malformation with extensive deep lymphatic involvement have been reported in the literature. There was a female predilection with reported involvement of the forearm, thigh, vulva, axilla, jaw, and even the tongue. The patients' ages ranged from infancy to adulthood, with some patients complaining of skin lesions and recurrent cellulitis for years, sometimes leading to sepsis and death. There is variable outcome for surgical intervention in such cases.
Conclusion
Mixed lymphatic malformation can present extensively affecting several parts of the body causing significant disfigurement with possible recurrence after surgical intervention.
Introduction
The lymphatic system is essential for regulating plasma volume and preventing tissue pressure buildup, as it reabsorbs a significant portion of the fluid and proteins that leak out the blood vasculature. Additionally, it supports cell nutrition and aids the immune response by directing antigen-presenting cells to lymph nodes. In the skin, lymphatic vessels serve as a critical exit route for Langerhans cells [1]. Anomalies in the development of lymphatic channels can lead to various diseases, including lymphatic malformation which can be either localized or diffuse and may be either congenital or acquired [2]. Notably, lymphatic malformation shows no variation across racial groups, with up to 90% of cases diagnosed before the age of two [3].
The International Society for the Study of Vascular Anomalies (ISSVA) established a comprehensive classification system that categorizes vascular anomalies based on their clinical characteristics and whether they are accompanied by other symptoms, which includes cystic lymphatic malformation, Kaposiform lymphangiomatosis, Gorham–Stout disease, and Channel-type lymphangioma. The cystic type is further categorized into macrocystic lymphatic malformation (diameter greater than 1cm), microcystic lymphatic malformation (diameter less than 1cm), formerly called lymphangioma circumscriptum, and mixed cystic lymphatic malformation. Histologically, the lymphatic cysts may either appear empty or contain a protein-rich fluid abundant in lymphocytes and macrophages [3].
Cutaneous lymphatic malformation represents only 4% of all vascular tumors. Microcystic lymphatic malformation is an uncommon superficial variant that primarily affects the axilla, groin, thighs, and buttocks. The “frog-spawn” appearance is due to the distinguished formation of dispersed clusters of small, thin-walled, translucent vesicles on the skin. Additionally, microcystic lymphatic malformation may contain a prominent vascular or hemorrhagic component, causing the lesion's color to vary from pink to red to black, depending on the proportion of blood to lymph within the vesicles. The present report aims to describe a case of mixed lymphatic malformation with extensive involvement, disfiguring various parts of the body [4].
The current study was written in line with CaReL guidelines and the credibility of references has been verified using Kscien’s list [5,6].
Case Presentation
Patient information
A 3.5-year-old male presented with complaints of intermittent bleeding from skin lesions in the right axillary region. Additionally, the patient complained of extensive swelling in the neck, axilla, and upper back, and swelling of the right middle finger. There was no family history of similar skin lesions or swelling. The patient's past surgical history was notable for herniorrhaphy and axillary mass resection.
Physical examination
On physical examination, there were multiple skin-colored compressible masses on the neck, axillae, trunk, and upper limbs. On the axillae and upper back, there were multiple small groups of papulovesicular lesions with characteristic “frog-spawn” appearance with many bleeding points. The patient had marked bilateral cervical swelling, extending laterally on both sides of the neck, with prominent bulges measuring approximately up to 10 cm on each side, that extended to involve the entire right upper limb down to the hand. Bilateral axillary swelling was observed, accompanied by a scar in the left axilla, indicative of previous surgical intervention. Additionally, swelling was evident in the entire middle and upper back, with a pronounced rounded bulge in the right upper back. The swelling extended across the back, with slight marbling noted on the overlying skin. There was also swelling of the chest, most prominently around the sternum. In addition, there was swelling in the right middle finger (Figure 1).
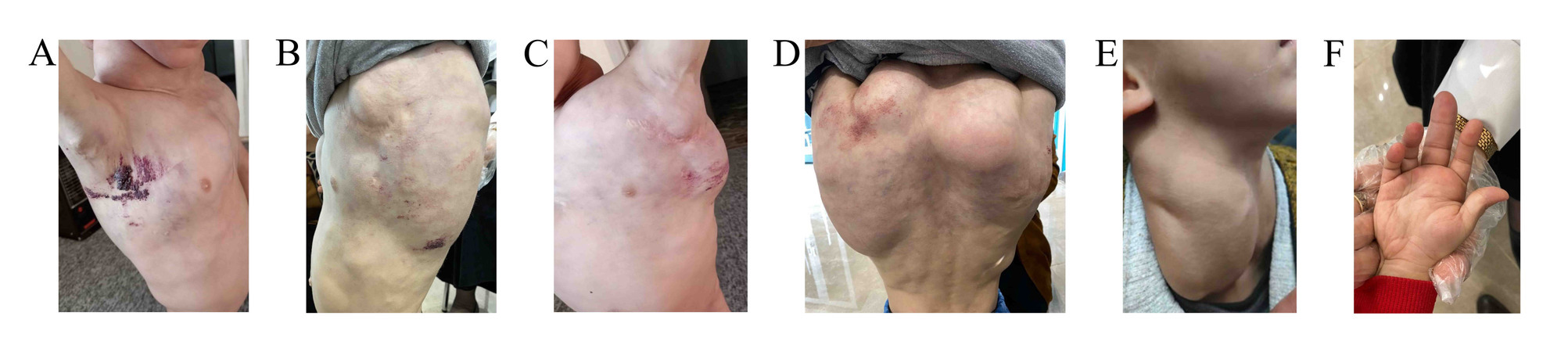
Diagnostic approach
The skin lesions were diagnosed as microcystic lymphatic malformation (lymphangioma circumscriptum) based on their location, the characteristic appearance of the vesicular lesions, and the history of oozing blood. The involvement of deeper lymphatic system swelling further supported the diagnosis.
The laboratory blood investigations including complete blood count, thyroid stimulating hormone, C-reactive protein, and D-dimer were all within the reference range. Ultrasonography of the axilla and inguinal regions showed fairly symmetric, bilateral axillary soft tissue lesions with ill-defined, heterogeneous echogenicity, measuring approximately 8×4 cm. Small cystic areas were present within the lesions, with minimal internal vascularity but no evidence of phleboliths or prominent vessels. The sonographic features were non-specific, and potential differentials included hibernoma, lipoblastoma, and atypical hemangioma. A left axillary lymph node measuring 13×8 mm was noted; however, the bulk of the swelling was attributed to the soft tissue mass. No enlarged inguinal lymph nodes were observed, but a bilateral large inguinal hydrocele was present, along with bilateral undescended testes located in the inguinal regions. The other abdominal organs showed no abnormalities.
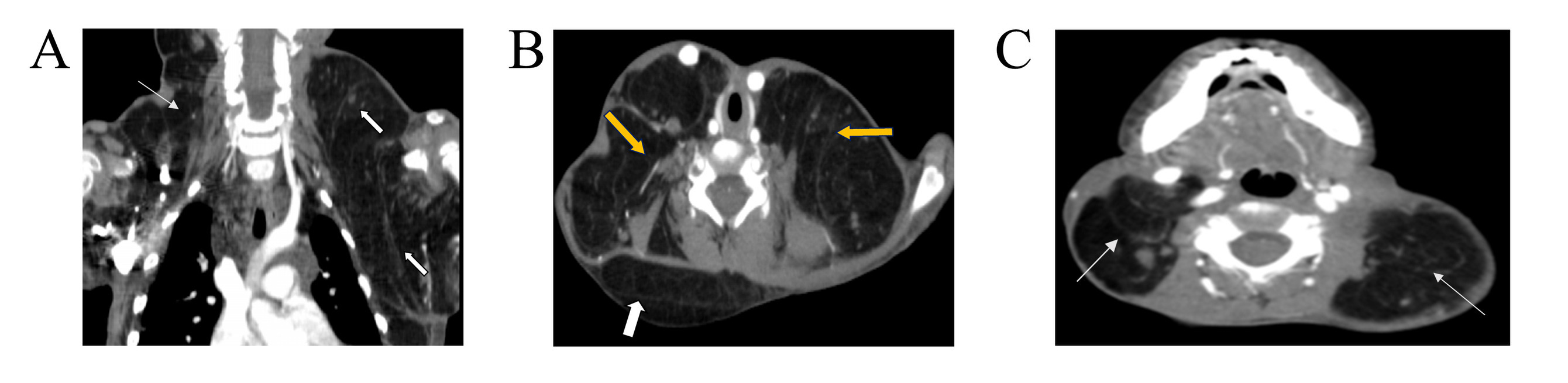
Contrast-enhanced computed tomography scan of the neck and upper chest showed normal lung parenchyma and a non-enhancing hypodense mass in the left axilla extending to the left supraclavicular region and a hypodense mass in the right side of the neck (Figure 2). The axillary magnetic resonance imaging (MRI) revealed bilateral symmetrical axillary masses, measuring 6×5×5 cm on the right and 6.5×5×4.5 cm on the left. These masses exhibited homogeneous high signal intensity on both T1 and T2-weighted images and were suppressed on fat-saturated sequences, with no enhancement post-contrast. The masses were associated with multiple enlarged axillary lymph nodes, the largest measuring 13×10 mm on the right and 13×9 mm on the left. Lipoblastoma was suggested as a differential, and biopsy was advised.
Biopsy of the axillary masses showed multiple fatty gray and tan pieces of tissue, the largest one measuring 85x80x40 mm. The microscopic sections showed multiple dilated cavernous and capillary size vascular spaces lined a by single layer of bland endothelium with flattened nuclei, surrounded by nodular aggregates of small mature lymphocytes and embedded in large lobules of mature adipose tissue, with areas that contain fetal-type skeletal muscle in process of maturation. There was no evidence of granulomata or invasive malignancy in the examined sections. Histopathology results were most consistent with macrocystic lymphatic malformation. A diagnosis of mixed lymphatic malformation was made.
Therapeutic intervention and follow-up
The patient previously underwent surgery to address the left axillary swelling; however, the lymphangioma recurred without significant improvement. Once-daily administration of sildenafil 10 mg and propranolol 10 mg yielded a good clinical response within the first three months, however on follow up there was an increase in the size of the mass. The frequency sildenafil dose was increased to 10 mg twice daily, and Pulsed Dye Laser (PDL) was applied for the microcystic lymphatic lesions for three sessions with no satisfactory response. At present, the patient is under observation with regular follow-up to monitor any potential complications or changes in the swellings that may require further intervention.
Discussion
The human skin comprises two distinct lymphatic plexuses. The superficial plexus, located near the subpapillary arterial network, consists of thin, non-valvular vessels extending into the dermal papillae. Lymph from this network drains into larger vessels situated in the lower dermis and the superficial subcutaneous tissue. The deep lymphatic plexus, positioned beneath the second arterial network, contains numerous valves and closely parallels the venous collecting system of the lower dermis [1].
The underlying pathophysiology of lymphatic malformation is characterized by fluid-filled muscular cisterns situated within the subcutaneous tissue, which establish a connection with the surface through dilated dermal lymphatic channels. Cutaneous involvement arises as a secondary consequence of the pressure exerted within the cisterns, driven by the pulsatile activity of their muscular walls. These cisterns do not communicate with the normal lymphatic system and lack any functional physiological purpose. The full extent of deep lymphangiomatosis cannot be accurately evaluated through physical examination alone, as the cisterns may extend significantly beyond the region occupied by the superficial vesicles [7]. For instance, a 13-year-old female reported by Palmer et al. complained of superficial lesions affecting the right inner thigh diagnosed as microcystic lymphangioma, but the right thigh had a 50cm thickness compared to the left side of 47.5cm. Further imaging revealed atypical expansion of the lymphatic channel stretching from the femoral region to the right para-aortic area [8]. Furthermore, a 15-year-old had more than 5 years of oozing from the microcystic lymphatic malformation affecting the left anteromedial inner thigh with deeper lymphatic involvement reaching the labia majora causing significant swelling and difficulty ambulating and disfigurement, during the literature review more cases have been identified with various parts of the body being affected (Table 1) [ 7-11].
|
Author/ year of publication |
Age (years) |
Sex |
Duration of complaint |
Microcystic lymphangioma location |
Deeper lymphatic involvement |
Diagnostic imaging |
Histopathology |
Treatment |
Follow-up |
Outcome |
|
Palmer et al./ 1978 [8] |
13 |
F |
Several years |
Right inner thigh |
Right thigh enlargement |
A lymphangiogram demonstrated abnormal dilation of the lymphatic channel extending from the femoral region up to the right para-aortic region |
Dilated endothelial lined lymph channels |
N/A |
N/A |
N/A |
|
Anees et al./ 2006 [9] |
15 |
F |
Six years |
Left inner thigh |
From the left groin up to the proximal half of left thigh, localized on the medial aspect, and involving the left labia majus |
N/A |
Dilated, large “cistern” of lymph vessels lined by a single layer of endothelium and extending to the deeper zone of the dermis. |
Surgical intervention |
1.5 years |
No recurrence |
|
Mordehai et al./ 1998 [10] |
1 month |
M |
Since birth |
Right forearm |
Right axilla |
Chest radiograph showing dilatation of upper mediastinum, right side
CT scan of chest showing cystic mass 3 cm in diameter in anterior mediastinum adherent to superior vena cava on right side of trachea |
Confirmatory of lymphangioma circumscriptum |
Wide excision of the axillary lymphangioma, right thoracotomy. |
9 years |
recurrent episodes of cellulitis of the right forearm and axilla with local recurrence of the lymphangioma in the form of small vesicles 1±2 mm in diameter along the scar tissue of previous suture lines and at the border of the skin grafts and lymphorrhea |
|
Rao et al./ 1998 [11] |
25 |
F |
Since birth |
Right temporal area, raised lesions over the right half of the tongue |
Right jaw |
N/A |
multiple dilated, cystic vessels in the papillary and sub-papillary dermis, containing lymph and occasional erythrocytes and were lined by a single layer of endothelium |
N/A |
N/A |
N/A |
|
Beard et al./ 1995 [7] |
9 |
F |
Since birth |
Buttocks and the stump of her right leg in the area of previous amputation |
Entire right lower extremity, extensive abdominopelvic lymphangiomatosis |
Radiographs revealed a soft tissue swelling without evidence of bony enlargement. lymphangiogram were most consistent with a lymphangiohemangioma |
Biopsy was consistent with lymphangioma circumscriptum |
amputation above the right knee at age four. Radiotherapy. frequent scissor excisions and cryosurgery. carbon dioxide laser ablation of the coccygeal, intergluteal, buttock, perineal, and perianal lymphangiomatosis |
Until the age of 15 |
Recurrent cellulitis, temporary improvement after interventions. The patient ultimately died at 15 after cellulitis, UTI, and resultant sepsis. |
The skin features of microcystic lymphangioma make its diagnosis straightforward but it can be misdiagnosed as other conditions, with a broad list of differential diagnoses including dermatitis herpetiformis, angiokeratoma circumscriptum, herpes simplex, herpes zoster, and hemangioma. In an interesting case by Juca et al, due to the verrucous appearance of the microcytic lymphangioma affecting a toe, leishmaniasis, skin tuberculosis, and warts were suspected first. However, histopathology was suggestive of microcystic lymphatic malformation showing convoluted ectatic lymph vessels in the papillary dermis [12]. Histopathology of the current patient revealed dilated vascular spaces lined by flat endothelium, surrounded by lymphoid aggregates, mature fat, and maturing fetal-type skeletal muscle.
Although lymphatic malformations are benign and typically a cosmetic concern, they can cause symptoms like burning, itching, and pain especially when complicated by infection, hemorrhage, or mechanical irritation. Leakage of blood-tinged, sometimes foul-smelling lymphatic fluid, either spontaneously or after minor trauma, can lead to discomfort and embarrassment. Open vesicles may also increase the risk of infection, leading to recurrent cellulitis and sometimes leading to sepsis and death. Due to cosmetic concerns, emotional distress, or frequent infections, patients often seek intervention [4,7]. Previously lymphangiogram was used to assess the extent of deeper lymphatic involvement as was the case by Palmer et al and Beard et al. Currently, computed tomography and magnetic resonance imaging are utilized more often [7-9]. For example, in the case report by Mordehai et al. computed tomography of a 1-month-old was shown to have a 3 cm cystic mass located in the anterior mediastinum, adjacent to the right side of the trachea and adherent to the superior vena cava. While on physical examination there was a group of vesicles on the right forearm with deeper cystic hygroma causing significant swelling of the right axilla. Interestingly, when wide excision of the axillary lymphangioma was carried out, the microcystic lymphatic malformation was then present at the site of the prior surgical site and suture lines with repeated episodes of cellulitis and lymphorrhea during the nine-year follow-up period [10]. Similarly, the current case had excision of the lymphangioma in the left axilla however, there was no recurrent cellulitis or vesicle formation at the scar site. The surgery was deemed ineffective as the deeper lymphatic involvement of the axilla recurred. Nonetheless, Surgical excision remains the primary treatment modality for the extensive/classical subtype, despite the potential for recurrence, as the surgical approach is predicated on the complete excision of all sequestrated lymphatic cisterns within the subcutaneous plane, as they are the principal etiological factor. The patient reported by Anees et al. resulted in good outcomes with no sign of recurrence 1.5 years after the operation of the medial left thigh. Other treatment modalities include intra-lesional sclerotherapy with doxycycline or Picibanil, as well as vaporization using carbon dioxide laser [9]. In a systematic review including 28 individuals with carbon dioxide laser ablation of microcystic lymphangioma, eight of them were disease-free and ten of them showed partial recurrence up to a three-year follow-up period, serving as an effective therapeutic option with a tolerable side-effect profile. Notably, it may also outperform surgical intervention for lesions with extensive surface area and deep involvement, though additional research is needed to confirm this advantage [4].
One limitation of our study is the absence of histopathological images, as only the histopathology reports were available. In addition, the relatively short follow-up period does not showcase the long-term physical and psychological outcomes of extensive mixed lymphatic malformation affecting several parts of the body.
Conclusion
Mixed lymphatic malformation can present extensively affecting several parts of the body causing significant disfigurement with possible recurrence after surgical intervention.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Written informed consent was obtained from the patient for publication.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: RSA and SOS were significant contributors to the conception of the study and the literature search for related studies. DH and JIH were involved in the literature review, study design, and manuscript writing. LJM, OMH, SOA, DHBMS and FJS were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. RSA and DH confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: ChatGPT-3.5 was used to assist in language editing and improving the clarity of the manuscript. All content was reviewed and verified by the authors. Authors are fully responsible for the entire content of their manuscript.
Data availability statement: Not applicable.
References
- Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. InJournal of Investigative Dermatology Symposium Proceedings 2000;5(1):14-19. doi:10.1046/j.1087-0024.2000.00001.x
- Fatima S, Uddin N, Idrees R, Minhas K, Ahmad Z, Ahmad R et al. Lymphangioma circumscriptum: clinicopathological spectrum of 29 cases. Journal of the College of Physicians and Surgeons Pakistan. 2015;25(9):658.
- Liu X, Cheng C, Chen K, Wu Y, Wu Z. Recent progress in lymphangioma. Frontiers in Pediatrics. 2021;9:735832. doi:10.3389/fped.2021.735832
- Savas JA, Ledon J, Franca K, Chacon A, Zaiac M, Nouri K. Carbon dioxide laser for the treatment of microcystic lymphatic malformations (lymphangioma circumscriptum): a systematic review. Dermatologic surgery. 2013;39(8):1147-57. doi:10.1111/dsu.12220
- Prasad S, Nassar M, Azzam AY, García-Muro-San José F, Jamee M, Sliman RK, et al. CaReL Guidelines: A Consensus-Based Guideline on Case Reports and Literature Review (CaReL). Barw Med J. 2024;2(2):13-19. doi:10.58742/bmj.v2i2.89
- Kakamad FH, Abdalla BA, Abdullah HO, Sami S. Omar, Shvan H. Mohammed, Sasan M. Ahmed, et al. Lists of predatory journals and publishers: a review for future refinement. Eur Sci Ed. 2024;50:e118119. doi:10.3897/ese.2024.e118119
- Beard G, Abernethy L, Callen JP. Lymphangioma circumscriptum associated with elephantiasis lymphangiomatosa. Journal of the European Academy of Dermatology and Venereology. 1995;5(3):275-8. doi:10.1016/0926-9959(95)00102-4
- Palmer LC, Strauch WG, Welton WA. Lymphangioma circumscriptum: A case with deep lymphatic involvement. Archives of Dermatology. 1978;114(3):394-6. doi:10.1001/archderm.114.3.394
- Anees A, Yaseen M, Sherwani R, Khan MA. Extensive Lymphangioma Circumscriptum. Journal, Indian Academy of Clinical Medicine. 2006;7(1).
- Mordehai J, Kurzbart E, Shinhar D, Sagi A, Finaly R, Mares AJ. Lymphangioma circumscriptum. Pediatric surgery international. 1998;13:208-10.
- Rao MV, Thappa DM, Ratnakar C. Lymphangioma circumscriptum of the skin and tongue associated with cystic hygroma. Indian Journal of Otolaryngology and Head & Neck Surgery. 1998;50(3):266. doi:10.1007/BF03007004
- Jucá NB, Crisóstomo MG, Oliveira LM, Cavalcante HA, Sousa AR. Acral microcystic lymphangioma: differential diagnosis in verrucous lesions of the extremities. Anais Brasileiros de Dermatologia. 2011;86:343-6. doi:10.1590/S0365-05962011000200020.

This work is licensed under a Creative Commons Attribution 4.0 International License.

