Echinococcus granulosus in Environmental Samples: A Cross-Sectional Molecular Study
Abstract
Introduction
Echinococcosis, caused by tapeworms of the Echinococcus genus, remains a significant zoonotic disease globally. The disease is particularly prevalent in areas with extensive livestock farming. Humans primarily acquire infection through consumption of contaminated food or water, often from environmental contamination by definitive host feces. This study aimed to detect the presence of E. granulosus DNA (deoxyribonucleic acid) in water and vegetable samples collected from Sulaymaniyah Governorate, Iraq.
Methods
A cross-sectional study was conducted in Sulaymaniyah Governorate, Iraq, in April 2025. Water and vegetable samples were collected from both urban and rural areas. DNA extraction was performed from all samples, and E. granulosus DNA was explored using a qPCR (quantitative polymerase chain reaction) assay. Sample processing included filtering water, washing vegetables, and DNA extraction under optimized conditions.
Results
A total of 245 samples, comprising 98 (40.0%) water samples and 147 (60.0%) vegetable samples, were analyzed, with 111 (45.3%) from urban and 134 (54.7%) from rural areas. Despite the comprehensive sampling, no E. granulosus DNA was detected in any sample. All control reactions yielded positive results, but no amplification was observed in the field samples, indicating the absence of E. granulosus contamination.
Conclusion
This study found no evidence of E. granulosus DNA in water or vegetable samples from Sulaymaniyah, Iraq, suggesting a low likelihood of environmental contamination in this region. but seasonal changes, the restricted sample size, and methodological limitations mean that the presence of contamination cannot be completely excluded.
Introduction
Echinococcosis is a globally prevalent zoonotic disease caused by the larval or adult stages of tapeworms belonging to the genus Echinococcus (family Taeniidae). Recognized as one of the oldest documented human infections, its history dates to Hippocrates. The two primary forms affecting humans are cystic echinococcosis, also known as hydatid disease or hydatidosis, caused by Echinococcus granulosus, and alveolar echinococcosis, caused by Echinococcus multilocularis [1].
Echinococcosis has a worldwide distribution, with higher prevalence reported in areas such as Eastern Europe, South Africa, the Middle East, South America, Australia, and the Mediterranean, where livestock farming is widespread [1]. In some communities, the human incidence exceeds 50 cases per 100,000 person-years, with prevalence rates ranging from 5% to 10%. The World Health Organization (WHO) identifies CE as one of the major foodborne parasitic diseases globally [2].
Domestic dogs are the main definitive hosts for both Echinococcus species and represent the greatest risk for transmitting cystic and alveolar echinococcosis to humans. Dogs acquire infection by consuming livestock offal containing hydatid cysts and subsequently shed parasite eggs in their feces, which contaminate the environment, including soil, water, and grazing areas. Livestock become infected by ingesting these eggs while grazing, and humans are typically infected through the consumption of contaminated food or water [1].
Hydatid cysts can develop in nearly any organ, though the liver is most commonly affected (approximately 75%), followed by the lungs (15%), with rare involvement of the brain (2%) and spine (1%). Early stages of infection are often asymptomatic, with clinical manifestations emerging only when cysts enlarge or become complicated [3].
Environmental contamination with Echinococcus eggs can be substantial in endemic areas with high definitive host prevalence, creating a risk for human infection. However, contamination through food and the environment has long been overlooked, even though ingestion of infective eggs from these sources is the primary route of human infection [4].
This study aimed to detect the presence of E. granulosus DNA (deoxyribonucleic acid) in water and vegetable samples collected from both urban and rural areas of Sulaymaniyah Governorate, Iraq, using a sensitive qPCR (quantitative polymerase chain reaction) assay. All references were assessed using trusted predatory journal lists to verify their scholarly integrity [5].
Methods
Study design
A cross-sectional study design was employed. Water and vegetable samples were collected in April 2025 from multiple sites across Sulaymaniyah Governorate, Iraq. Sampling sites were randomly selected across the area to ensure the representativeness of both urban and rural areas. Sampling was conducted under sterile conditions, and all collected material was immediately transferred to the molecular biology department at Smart Health Tower for laboratory processing.
Sample collection
Water samples were collected directly from rivers, irrigation canals, and storage sources into sterile polypropylene containers. For each randomly selected site, one liter of water was obtained using sterile polypropylene containers. Vegetable samples, including commonly consumed leafy greens and root crops. Approximately 200 g per sample was collected using sterile gloves and placed in clean polyethylene bags. All containers and bags were clearly labeled with the date, site of collection, and sample type. Samples were maintained at 4–8 °C in insulated boxes with ice packs and processed within 6 h of collection.
Sample processing
Upon arrival at the laboratory, water samples were filtered through sterile membrane filters to concentrate parasitic elements. The residues retained on the filters were rinsed with sterile phosphate-buffered saline (PBS) (pH 7.4) and collected for downstream analysis. Vegetable samples were washed by agitation in 500 mL sterile PBS for 10 minutes at 150 rpm. The wash solutions were collected and centrifuged at 3,000 × g for 10 minutes. Resulting pellets were retained for downstream molecular examination.
To enhance DNA extraction efficiency, the pellets were subjected to mechanical disruption using a sterile mortar and pestle under liquid nitrogen, followed by three freeze–thaw cycles between liquid nitrogen (–196 °C) and a 37 °C water bath. This process facilitated rupture of eggshells or larval teguments, releasing nucleic acids for downstream extraction. The homogenate was then processed immediately for DNA extraction.
DNA extraction
Genomic DNA was extracted using the PureLink Genomic DNA Mini Kit (K1820-01, Invitrogen, Thermo Fisher Scientific, USA) according to the manufacturer’s instructions, with modifications adapted for E. granulosus. The procedure included sequential steps of lysis, binding, washing, and elusion. During the lysis step, the processed samples were incubated with Genomic Lysis/Binding Buffer and Proteinase K at 55–60 °C until complete digestion was achieved. RNase A was added during this stage to eliminate RNA contaminants. Following lysis, ethanol was added to the lysates, which were then transferred to silica-based spin columns. The washing step involved sequential treatment with PureLink Wash Buffer 1 and Wash Buffer 2 to remove salt, proteins, and other contaminants. A final dry spin was performed to eliminate residual ethanol. For elusion, PureLink Genomic Elution Buffer was added to the columns, and DNA was released into sterile collection tubes following centrifugation.
DNA quality assessment
The quality and concentration of the extracted DNA were evaluated using a spectrophotometer (NanoDrop, Thermo Fisher Scientific, USA). Absorbance was measured at 260 nm and 280 nm to determine purity. Purity assessment was performed by calculating the A260/A230 ratio, with acceptable values ranging between 1.9 and 2.2. DNA integrity was further confirmed by electrophoresis on a 1% agarose gel prepared in 1X Tris-borate-EDTA buffer and stained with ethidium bromide. Bands were visualized under ultraviolet illumination, and intact high-molecular-weight bands were taken as indicators of high-quality genomic DNA.
Preparation of primers and probes
Primers and probes were prepared according to the manufacturer’s instructions (Sigma-Aldrich). Stock solutions for each primer and probe were resuspended in nuclease-free water, briefly vortexed, and centrifuged. Working solutions were prepared by diluting the stock solutions 1:10 in nuclease-free water.
qPCR reaction mixture preparation
Luna Universal Probe qPCR Master Mix and other reaction components were thawed at room temperature and then placed on ice. Each component was briefly mixed by inversion and gentle vortexing. Reaction mixtures were prepared for the required number of samples, including 10% overage. For each 20 µL reaction, the following components were combined: 10 µL Luna® Universal Probe qPCR Master Mix (2X), 1 µL Cox3 forward primer (10 µM), 1 µL Cox3 reverse primer (10 µM), 0.5 µL Cox3 probe-FITC (10 µM), 1 µL template DNA (20–100 ng/µL), and 6.5 µL nuclease-free water (Table 1) [6,7]. The mixture was gently mixed and centrifuged briefly to collect the solution at the bottom of the tube. Aliquots were dispensed into qPCR tubes or plates, and DNA templates were added. Tubes or plates were spun briefly at 2,500–3,000 rpm to remove bubbles.
|
Target Species |
Target gene |
Primer and probe |
GenBank reference |
Oligonucleotide sequence (5’–3’) |
Target size (bp) |
Reference |
|
Echinococcus granulosus s.s |
Cytochrome oxidase subunit III |
Eg_cox3_Forward |
AF297617.1(UK) (Le et al. 2002) [7] |
TATCTGTAACACCACAAAACTCAAACC |
149 | Knapp et al. 2023 [6] |
|
Eg_cox3_Reverse |
CGTTGGAGATTCCGTTTGTTG |
|||||
|
Eg_cox3_Probe |
AACAAAAGCAAATCACAACAACGTCAACCC |
qPCR cycling conditions
Real-time qPCR was performed using the following thermal profile: initial denaturation at 95 °C for 60 seconds, followed by 40–45 cycles of 95 °C for 15 seconds and 60 °C for 30 seconds, with fluorescence readings collected at the extension step in the Fluorescein Isothiocyanate (green) channel.
Data analysis and standard curve
qPCR data were analyzed according to the instrument manufacturer's instructions. Standard curves were generated by plotting the logarithm of input DNA concentrations against Cq values to determine reaction efficiency, which was considered acceptable between 90–110% (slope –3.6 to –3.1). Correlation coefficients (R²) ≥ 0.98 were accepted. Specificity was verified by ensuring a Cq difference of ≥3 between template-containing and non-template controls. Method detection limits were determined using eight serial dilutions of the initial DNA concentration (100 ng/µL) to assess assay sensitivity. Samples were evaluated relative to standard curves and control reactions, with appropriate dilution factors accounted for.
Results
A total of 245 samples were collected and analyzed, comprising 98 (40.0%) water samples and 147 (60.0%) vegetable samples. Of these, 111 (45.3%) were obtained from urban areas and 134 (54.7%) from rural areas. Geographically, 119 (48.6%) samples were collected from the eastern region, 21 (8.6%) from the western region, 56 (22.8%) from the northern region, and 49 (20.0%) from the southern region of Sulaymaniyah Governorate (Table 2).
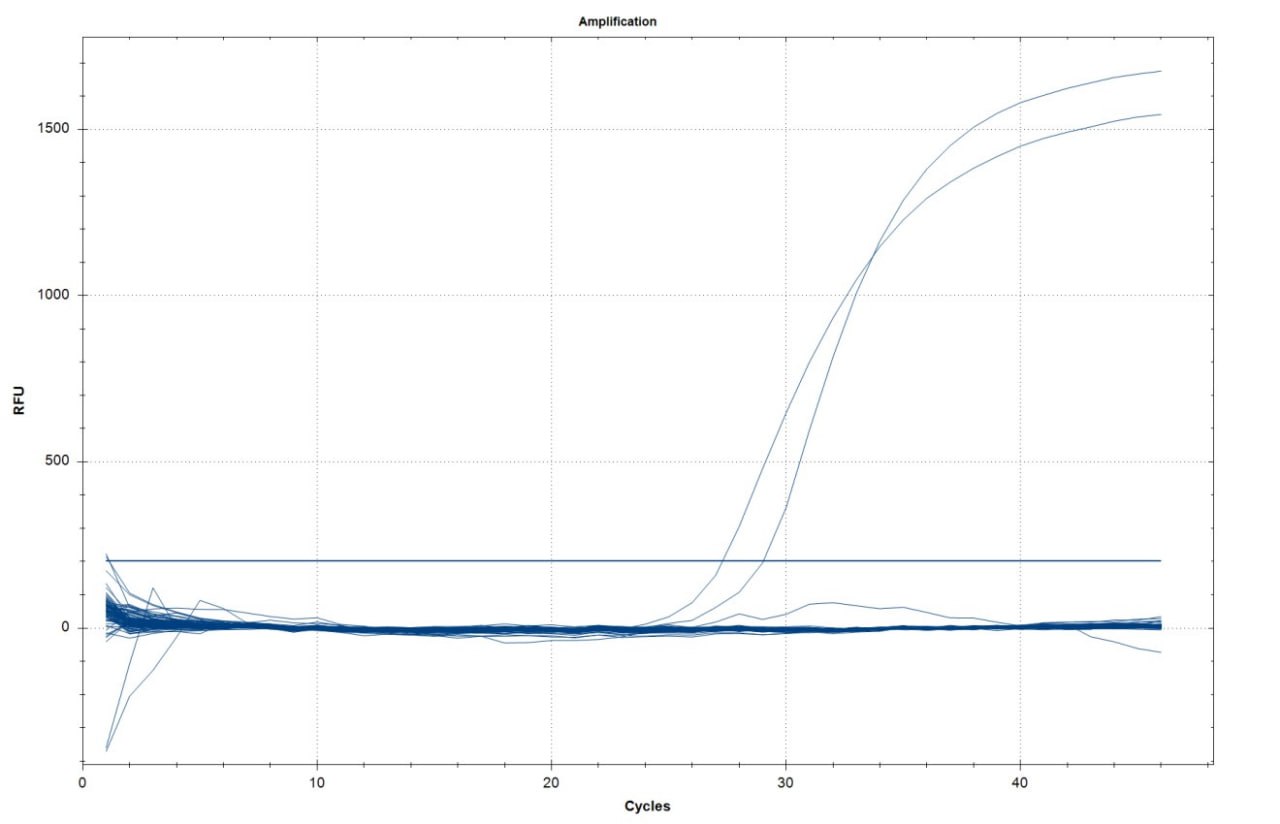
The qPCR assay demonstrated clear amplification in the positive controls, which produced sharp exponential fluorescence curves crossing the threshold between cycles 28 and 30. In contrast, all field samples, including both water and vegetables, exhibited flat baseline fluorescence with no detectable signal above the threshold, confirming the absence of amplification (Figure 1). Therefore, none of the 245 field samples tested positive for E. granulosus DNA.
|
Parameters |
Frequency (%) |
|
Residence |
|
|
Urban |
111 (45.3) |
|
Rural |
134 (54.7) |
|
Sample type |
|
|
Water |
98 (40.0) |
|
Vegetables |
147 (60.0) |
|
Region |
|
|
East |
119 (48.6) |
|
West |
21 (8.6) |
|
North |
56 (22.8) |
|
South |
49 (20.0) |
Discussion
Fresh vegetables are a vital part of a nutritious diet, but they may also act as carriers of protozoan cysts and helminth eggs or larvae. The moist conditions required for their growth create a favorable environment for the persistence and transmission of enteroparasitic forms. In many developing areas, the use of irrigation water contaminated with human or animal feces has been recognized as a key factor contributing to high levels of vegetable contamination with helminth eggs [8].
In this cross-sectional study of 245 water and vegetable samples collected from urban and rural areas of Sulaymaniyah, Iraq, no E. granulosus DNA was detected by qPCR. This absence of positive findings contrasts with reports from other regions documenting occasional environmental contamination. For example, Barosi and Umhang (2024) reported the presence of E. granulosus and E. multilocularis eggs in several environmental matrices, including water, soil, vegetables, and berries, with prevalence rates varying considerably [4]. Similarly, the international multicenter MEmE project detected E. granulosus sensu lato DNA in 1.3% of lettuce samples across Europe, with much higher levels reported in certain non-European countries, such as 12% of lettuce in Tunisia [9]. In that study, all positive samples were identified as E. granulosus sensu stricto, the genotype commonly associated with sheep, which confirmed that parasite eggs can adhere to food crops. The same project also recorded particularly high contamination rates in berries, with E. granulosus sensu stricto DNA found in 12% of blueberries from Pakistan and in 81.3% of strawberries from Tunisia [9]. In contrast, this study’s samples yielded no positives, suggesting that contamination of water and vegetables with E. granulosus eggs in Sulaymaniyah may be substantially lower than in the regions surveyed elsewhere.
Regional comparisons also demonstrate variability. In Iran, a neighboring country where hydatidosis is endemic, field surveys using microscopic egg detection have identified taeniid eggs (which include E. granulosus) on fresh produce at notable rates. A large Iranian study reported contamination in 7.8% of more than 2,700 vegetable samples, with lettuce showing the highest frequency [10]. In Shiraz, located in southern Iran, taeniid eggs were detected in 2.5% (2/80) of vegetables from markets and in 4.1% (6/144) of those collected directly from farms [11]. In Qazvin Province, contamination was found in 1.8% of 218 vegetable samples, again involving Taenia/Echinococcus eggs [12]. However, all these surveys relied on microscopy and did not specifically confirm E. granulosus by molecular methods; to date, no study has definitively documented E. granulosus DNA in Iranian produce despite the microscopy-based evidence [13]. In Turkey, environmental investigations have focused mainly on definitive hosts. For example, one survey of red fox feces reported 0.5% positivity for E. granulosus [14].
A few studies outside the Middle East provide context for our negative results. Awosanya et al. (2022) detected E. granulosus sensu lato DNA in environmental samples from Nigeria, with 2% of irrigation water and 7% of soil samples testing positive by PCR [15]. In Japan, Mori and colleagues analyzed river water using environmental DNA methods and detected E. multilocularis DNA in only 0.78% of samples [16], showing that such approaches often yield very low detection rates even in endemic regions. Taken together, these studies indicate that although Echinococcus eggs can contaminate water and crops, detection rates are often low unless contamination levels are substantial.
One possible explanation for the absence of positive findings in this study is the ecological and agricultural context. Environmental conditions in Sulaymaniyah, including climate, farming practices, and seasonality, may have reduced the likelihood of contamination. Experimental studies show that E. granulosus eggs can survive for more than 200 days at 7 °C under humid conditions, about 50 days at 21 °C with low humidity, and only a few hours in hot, dry conditions around 40 °C. Temperature and humidity strongly influence egg infectivity, and the eggs are highly sensitive to desiccation [17]. In Iraqi Kurdistan, late April corresponds to spring, with moderate average temperatures (~22 °C) that could support egg survival. However, rainfall or irrigation may wash eggs away, and dry days may accelerate desiccation. Agricultural practices may also play a role. For instance, if vegetables in Sulaymaniyah (such as irrigated greens) are cultivated and washed in ways that limit contact with dog feces, contamination would be less likely. In addition, the use of treated water or protected cultivation methods would further reduce exposure risk. In contrast, the high detection rates reported in countries such as Tunisia and Pakistan may reflect open-field agriculture combined with climatic or hygiene conditions more favorable to egg persistence.
This study has several important limitations. First, the sample size of 245 and the sampling strategy may have been insufficient to detect low-prevalence contamination. Although 245 samples represent a moderate number, environmental egg contamination could be so sparse that even this number yielded no positives. Second, sampling was conducted at a single time point at the end of April, so seasonal variations were not captured. E. granulosus transmission can be seasonal; for example, dog infections may peak after livestock slaughter in winter. Therefore, a late-spring snapshot may have missed periods of higher contamination or, conversely, periods when spring rains already washed eggs away.
Geographically, the study was confined to Sulaymaniyah governorate, encompassing both urban and rural areas. This region may not represent the entire area of Iraqi Kurdistan or Iraq, or neighboring provinces. As a result, the negative findings cannot be generalized to broader regions or other habitat types.
Despite these limitations, the results are reassuring from a public health perspective, suggesting that under current conditions, contaminated water and vegetables may not constitute a significant route of E. granulosus transmission in Sulaymaniyah. However, other transmission routes, particularly direct contact with infected dogs or handling of contaminated soil, remain relevant. Preventive strategies should therefore continue to focus on regular deworming of dogs, safe disposal of livestock offal, public education on hand and food hygiene, and improvements in slaughterhouse sanitation [1].
Future studies should expand sampling to cover different seasons, larger sample sizes, and additional environmental matrices such as soil and dog feces. Incorporating assays for egg viability alongside qPCR would help determine whether detected DNA reflects viable, infectious eggs or degraded material. Integrating environmental monitoring with veterinary and human epidemiological data will provide a more comprehensive understanding of local transmission dynamics, ensuring that cystic echinococcosis control efforts in Sulaymaniyah remain evidence-based and effective.
Conclusion
No E. granulosus DNA was detected in the water and vegetable samples collected from Sulaymaniyah, Iraq. These results indicate a low likelihood of environmental contamination during the study period, but seasonal changes, the restricted sample size, and methodological limitations mean that the presence of contamination cannot be completely excluded.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Not applicable
Source of Funding: Smart Health Tower.
Role of Funder: The funder remained independent, refraining from involvement in data collection, analysis, or result formulation, ensuring unbiased research free from external influence.
Acknowledgements: None to be declared.
Authors' contributions: RQS and MNH were major contributors to the study's conception and to the literature search for related studies. HAN, AMM, and MMA were involved in the literature review, study design, and writing of the manuscript. SJQ, AKH, SAF, SMA, SSA, YMM and KKM were involved in the literature review, the study's design, and data collection. TOM, SLE, and HSA were involved in the literature review, the study's design, the critical revision of the manuscript, and the table processing. All authors have read and approved the final version of the manuscript. RQS and HAN confirm the authenticity of all the raw data.
Use of AI: ChatGPT-4.0 was used to assist in language editing and improving the clarity of the manuscript. All content was reviewed and verified by the authors. Authors are fully responsible for the entire content of their manuscript.
Data availability statement: Not applicable.
References
- Kakamad FH, Anwar KA, Ahmed HK, Habibullah IJ, Kaka Ali HH, Nasralla HA, et al. Risk factors associated with human echinococcosis: a systematic review and meta-analysis. Frontiers in Veterinary Science. 2024;11:1480579. doi:10.3389/fvets.2024.1480579
- World Health Organization. Echinococcosis. 2021 [cited 2025 Aug 20]. Available from: https://www.who.int/news-room/fact-sheets/detail/echinococcosis.
- Nasralla HA, Abdalla BA, Abdullah HO, Ahmed SM, Kakamad FH, Mohammed SH, et al. Current Perspectives on Cystic Echinococcosis: A Systematic Review. Judi Clin. J. 2025;1(1):12-26. doi:10.70955/JCJ.2025.1
- Barosi R, Umhang G. Presence of Echinococcus eggs in the environment and food: a review of current data and future prospects. Parasitology. 2024;151(13):1416-1431. doi:10.1017/S0031182024000945
- Kakamad FH, Abdalla BA, Abdullah HO, Sami S. Omar, Shvan H. Mohammed, Sasan M. Ahmed, et al. Lists of predatory journals and publishers: a review for future refinement. Eur Sci Ed. 2024;50:e118119. doi:10.3897/ese.2024.e118119
- Knapp J, Lallemand S, Monnien F, Felix S, Courquet S, Umhang G, et al. Real-time multiplex PCR for human echinococcosis and differential diagnosis. Parasite. 2023;30:3. doi:10.1051/parasite/2023003
- Le TH, Pearson MS, Blair D, Dai N, Zhang LH, McManus DP. Complete mitochondrial genomes confirm the distinctiveness of the horse-dog and sheep-dog strains of Echinococcus granulosus. Parasitology. 2002;124(1):97-112. doi:10.1017/S0031182001008976
- Adanir R, Tasci F. Prevalence of helminth eggs in raw vegetables consumed in Burdur, Turkey. Food Control. 2013;31(2):482-4. doi:10.1016/j.foodcont.2012.10.032
- Umhang G, Bastien F, Cartet A, Ahmad H, Van Der Ark K, Berg R, et al. Detection of Echinococcus spp. and other taeniid species in lettuces and berries: Two international multicenter studies from the MEmE project. International Journal of Food Microbiology. 2025;430:111059. doi:10.1016/j.ijfoodmicro.2025.111059
- Hajipour N, Soltani M, Ketzis J, Hassanzadeh P. Zoonotic parasitic organisms on vegetables: Impact of production system characteristics on presence, prevalence on vegetables in northwestern Iran and washing methods for removal. Food Microbiology. 2021;95:103704. doi:10.1016/j.fm.2020.103704
- Asadpour M, Malekpour H, Jafari A, Bahrami S. Diversity of parasitic contamination in raw vegetables commonly consumed in Shiraz, Southwest of Iran. Asian Pacific Journal of Tropical Disease. 2016;6(2):160-2. doi:10.1016/S2222-1808(15)61004-0
- Shahnazi M, Jafari-Sabet M. Prevalence of parasitic contamination of raw vegetables in villages of Qazvin Province, Iran. Foodborne pathogens and disease. 2010;7(9):1025-30. doi:10.1089/fpd.2009.0477
- Borhani M, Fathi S, Darabi E, Jalousian F, Simsek S, Ahmed H, et al. Echinococcoses in Iran, Turkey, and Pakistan: old diseases in the new millennium. Clinical microbiology reviews. 2021;34(3):10-128. doi:10.1128/cmr.00290-20
- Gürler AT, Gori F, Bölükbas CS, Umur Ş, Açıcı M, Deplazes P. Investigation of Echinococcus multilocularis in environmental definitive host feces in the Asian and the European parts of Turkey. Frontiers in Veterinary Science. 2018;5:48. doi:10.3389/fvets.2018.00048
- Awosanya EJ, Olagbaju A, Peruzzu A, Masu G, Masala G, Bonelli P. Detection of Echinococcus granulosus sensu lato in Environmental Samples from Ibadan, Oyo State, South West Nigeria. Veterinary Sciences. 2022;9(12):679. doi:10.3390/vetsci9120679
- Mori K, Imamura A, Hirayama I, Minamoto T. Detection of Echinococcus multilocularis in repurposed environmental DNA samples from river water. PeerJ. 2023;11:e15431. doi:10.7717/peerj.15431
- Aziz HM, Hama AA, Salih MA, Ditta A. Prevalence and molecular characterization of Echinococcus granulosus sensu lato eggs among stray dogs in Sulaimani Province—Kurdistan, Iraq. Veterinary sciences. 2022;9(4):151. doi:10.3390/vetsci9040151

This work is licensed under a Creative Commons Attribution 4.0 International License.

