Vol 4, No 1 (2026): Current Issue (Volume 4, Issue 1), 2026
Editorial
Evolving Challenges in Modern Qualitative Research
Snur Othman
Qualitative research works at revealing the depth of human experiences, cultural nuances, and complex social dynamics, yet it confronts formidable challenges including pervasive researcher subjectivity, methodological inconsistencies, ethical intricacies, resource burdens, data management overload, and struggles with establishing rigor and transferability that often invite skepticism from quantitative paradigms. These obstacles not only complicate the research process but also threaten the perceived validity and broader applicability of findings in fields like health, education, and social sciences. Addressing them requires deliberate strategies to fortify qualitative inquiry's contributions to knowledge [1].
Subjectivity and Researcher Bias
The interpretive essence of qualitative research inherently invites researcher bias, as personal worldviews, cultural backgrounds, and preconceptions influence every stage from question formulation to data interpretation. For example, during thematic analysis of interviews, a researcher's emphasis on certain participant quotes might overlook contradictory evidence, leading to unbalanced narratives. Mitigation strategies like reflexivity where researchers explicitly document their influences and triangulation, cross-verifying data from multiple sources, prove essential, though full elimination of subjectivity remains impractical in this paradigm [2].
Methodological Design and Rigor Hurdles
Crafting a robust qualitative design demands precise alignment between philosophical underpinnings, research questions, and methods such as phenomenology, grounded theory, or discourse analysis, yet mismatches frequently occur due to insufficient expertise. Determining data saturation when new data yields no fresh insights relies on subjective judgment, complicating claims of completeness, while ensuring transferability to other contexts necessitates detailed "thick descriptions" of participants and settings. In health research, these issues amplify without clear audit trails, prompting calls for standardized rigor criteria akin to quantitative benchmarks [3].
Data Collection and Management Complexities
Gathering qualitative data through prolonged interviews, focus groups, or ethnographies generates vast, unstructured volumes of transcripts, field notes, and multimedia that overwhelm storage, organization, and preliminary sorting. Logistical barriers, like recruiting hard-to-reach participants or adapting to virtual formats, further delay progress, while ensuring consistency across sessions proves elusive without rigid protocols. Digital tools offer relief for transcription and initial coding, but they demand technical proficiency and risk diluting contextual richness if misapplied [1-4].
Analysis and Interpretation Demands
Transforming raw qualitative data into coherent themes involves iterative coding, pattern identification, and narrative synthesis, a labor-intensive process prone to interpretive drift among team members. Balancing depth with transparency challenges researchers, especially when handling ambiguous or contradictory data, and emerging AI aids accelerate this but introduce concerns over algorithmic bias eroding human insight. Peer debriefing, inter-coder reliability checks, and software like NVivo enhance trustworthiness, yet the time investment often months strains projects and underscores the need for advanced training [4].
Ethical, Practical, and Interdisciplinary Tensions
Ethical navigation intensifies in qualitative work due to intimate participant interactions, raising issues like securing ongoing consent, safeguarding anonymity in sensitive topics, and managing power imbalances with vulnerable groups. Practical constraints, including high costs for fieldwork and participant fatigue, compound these, while interdisciplinary skepticism particularly from STEM fields questions replicability and generalizability. Mixed-methods integration and decolonial approaches that center marginalized voices offer bridges, but they require institutional support and evolved review board processes [5].
Emerging Trends and Solutions
Technological innovations like AI-driven analysis and big data integration promise efficiency, yet they challenge traditional methodological purity and amplify ethical risks around data privacy. Postqualitative and indigenous methodologies push boundaries by rejecting linear processes, fostering inclusivity amid globalization. Researchers advance by prioritizing comprehensive training, open-access protocols for auditability, and collaborative networks to elevate qualitative work's stature and impact.
Conflicts of interest: The author has no conflicts of interest to disclose.
Original Articles
Could first-trimester bleeding affect a newborn's Apgar score?
Leila Sekhavat, Atiyeh Javaheri
Abstract
Introduction
Vaginal bleeding is a common complication during pregnancy and may contribute to adverse pregnancy outcomes. This study aimed to evaluate the effect of first trimester bleeding on newborns Apgar scores.
Methods
A retrospective study was conducted on pregnant women who delivered at Shahid Sadoughi hospital in Yazd, Iran, between 2022 and 2023. Only singleton, nulliparous, non-diabetic women were included. Participants were divided into two groups: the exposure group (Bleeding Group) and control Group (Non-Bleeding Group), based on archived records. Apgar scores recorded at the first and fifth minutes after birth in newborns file were compared between groups.
Results
A total of 992 women were included, with 218 in the exposure and 774 in the control groups. The incidence of a first-minute Apgar score <7 was significantly higher in the bleeding group compared to controls (22.5% vs. 6.2%, p = 0.02). However, there was no significant difference in five-minute Apgar scores between groups.
Conclusion
This study demonstrated a positive association between first-trimester vaginal bleeding and a low first-minute Apgar score in newborns.
Introduction
The first trimester of pregnancy is a crucial period of fetal development, during which the body undergoes significant physiological changes to support the growing baby [1]. Maternal and fetal well-being are closely interconnected, and multiple factors influence fetal growth and metabolic programming.
First trimester bleeding defined as vaginal bleeding occurring between conception and 12 weeks of gestation. It is common and affecting between 16 - 25% of all pregnancies and often causes anxiety for both patients and clinicians [2-4]. Although many pregnancies with first-trimester bleeding progress without complication, emerging evidence suggests an increased risk of neonatal complications later in pregnancy [5,6]. The Apgar score, developed by Dr. Virginia Apgar in 1952, is a rapid and reliable method of assessing newborn condition and clinical status immediately after delivery [7-9]. The score is reported at one and five minutes after birth and, if below 7, at five-minute intervals up to 20 minutes [10]. Approximately 1% of low-risk live births have a five-minute Apgar score below 7, which is associated with a significantly higher risk of neonatal morbidity and mortality [11]. Low Apgar scores have also been linked to long-term adverse outcomes such as epilepsy, cerebral palsy, and developmental delays [12-14].
Numerous studies have investigated the relationship between first-trimester bleeding and neonatal health. Some of these studies indicate a correlation between first-trimester bleeding and low Apgar scores at the first and fifth minutes after birth [5,15-17]. Conversely, other studies have reported that first-trimester bleeding has no effect on newborn Apgar scores [18-21].
Several studies have explored the association between first-trimester bleeding and neonatal outcomes, with mixed results. Some found a correlation between early bleeding and low Apgar scores [5,15–17]. The others reported no significant relationship [18–21]. Some evidence suggests that only when bleeding results in complications such as intrauterine growth restriction (IUGR), preterm birth, or low birth weight does it significantly affect neonatal Apgar scores [5,22,23].
This study aims to further investigate the relationship between first-trimester bleeding and neonatal Apgar scores.
Methods
Study design and setting
This study was designed as a retrospective study. Data were collected from archived files at the hospital and medical records of pregnant individuals who delivered at Shahid Sadoughi hospital in Yazd, Iran, during one year.
Inclusion criteria
This study included singleton pregnancies that resulted in the delivery of live newborns at or beyond 37 weeks of gestation, with a birth weight greater than 2500 grams.
Exclusion criteria
Pregnancies were excluded if there were fetal anomalies, chronic maternal diseases such as diabetes mellitus, hypertension, renal, cardiac, or endocrine disorders, or any history of smoking or drug abuse. Additional exclusions included surgical conditions during pregnancy, multiple gestations, placental abruption or placenta previa in the later trimesters, and cases with incomplete medical records.
Grouping and data collection
Participants were categorized into two groups: an exposure group, consisting of pregnancies complicated by first-trimester vaginal bleeding, and a control group, comprising pregnancies without first-trimester bleeding. All included women were under 40 years of age. Demographic characteristics such as occupation, economic status, educational level, and maternal body mass index (BMI) were obtained. Clinical data were extracted from archived hospital records and patients' files, including obstetric history and detailed documentation of any first-trimester bleeding episodes. First-trimester vaginal bleeding was defined as bleeding occurring before 12 weeks of gestation in the presence of a closed cervix and a viable intrauterine pregnancy. Newborn outcomes, including Apgar scores at one and five minutes, were also retrieved from medical records. An Apgar score <7 at either time point was considered low, with scores classified as normal (>7), low (5–7), or very low (<5).
Statistical analysis
Data were analyzed using SPSS version 20. Continuous variables were compared using the t-test, while categorical variables were assessed using the chi-square test. A p-value of <0.05 was considered statistically significant.
Results
A total of 992 term singleton pregnancies were included in the analysis, comprising 218 women in the exposure (bleeding) group and 774 in the control group. Maternal and neonatal characteristics of both groups are presented in (Table 1).
|
Maternal characteristics |
Exposure group (first trimester bleeding) N = 218 |
Control group (without bleeding) N= 774 |
P-value |
|
Age in years N (%) <20 20 – 30 31 – 40 |
45 (20.6) 146 (67) 27 (12.4) |
155 (20) 513 (66.3) 106 (13.7) |
0.2 |
|
Body mass index (kg/m2) N (%) <18 18– 25 >25 |
38 (17.4) 145 (66.5) 35 (16.1) |
148 (19.1) 490 (63.3) 136 (17.6) |
0.1 |
|
Employment N (%) Yes No |
98 (44.9) 120 (55.1) |
379 (49) 395 (51) |
0.7 |
|
Educational level < 12 >12 |
66 (30.3) 152 (69.7) |
241 (31.1) 533 (68.9) |
0.4 |
|
Prenatal care Adequate Inadequate |
99 (45.4) 119 (54.6) |
363 (46.9) 411 (53.1) |
0.3 |
Newborns in the bleeding group had a significantly higher proportion of first-minute Apgar scores <7 compared with the control group (22.5% vs. 6.2%, p = 0.02). Although five-minute Apgar scores <7 were also more common among the bleeding group (8.7% vs. 6.7%), this difference did not reach statistical significance (p = 0.6) (Table 2).
| Neonatal Apgar score |
Exposure group (first trimester bleeding) N = 218 |
Control group (without bleeding) N= 774 |
P-value |
|
First min APGAR scores N (%) <7 >7 |
49 (22.5) 169 (77.5) |
48 (6.2) 726 (93.8) |
0.02 |
|
5 min after birth APGAR scores N (%) <7 >7 |
19 (8.7) 199 (91.3) |
52 (6.7) 722 (93.3) |
0.6 |
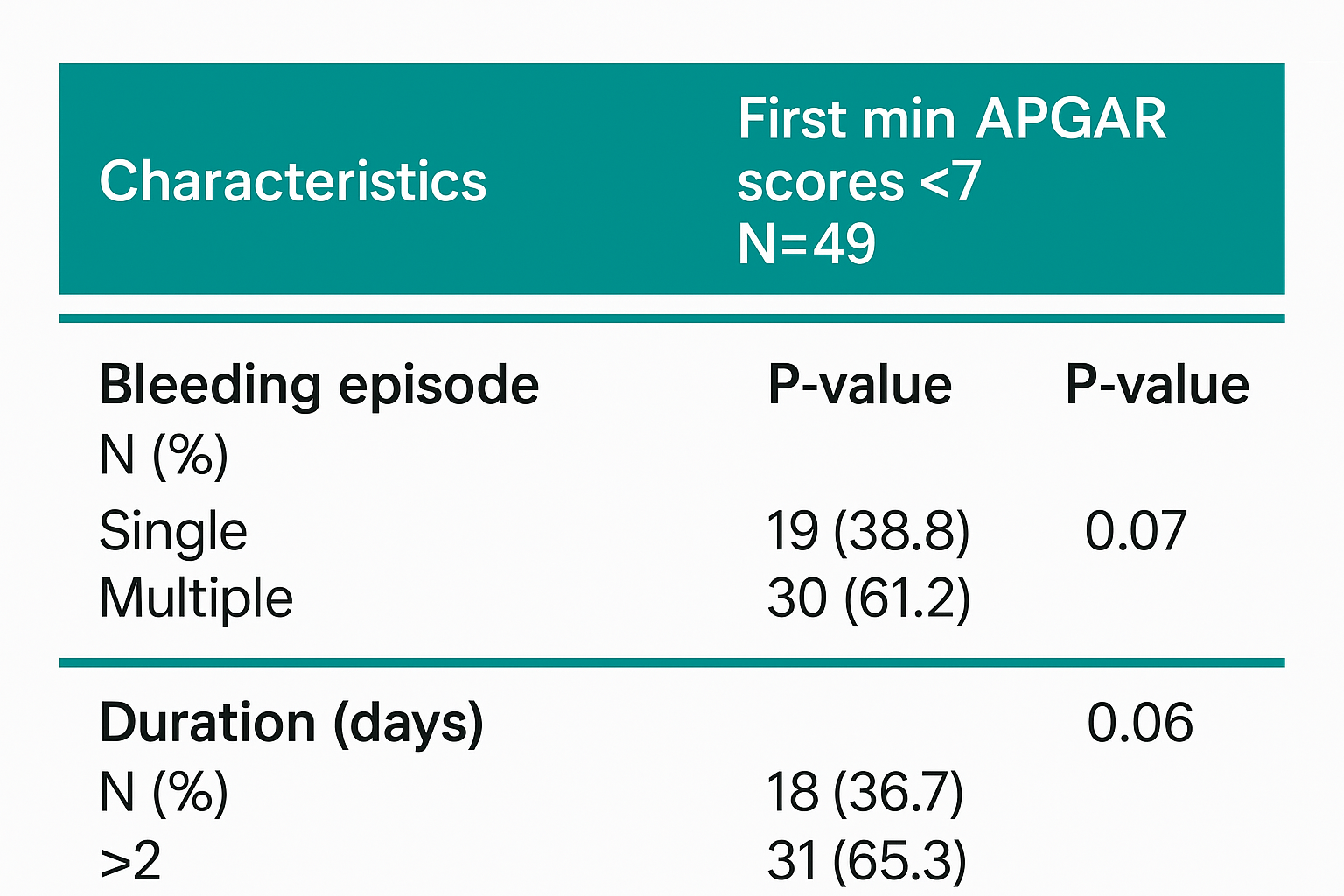
Women who experienced bleeding lasting more than two days had a greater frequency of low first-minute Apgar scores (65.3%) than those with shorter-duration bleeding (36.7%); however, this trend was not statistically significant (p = 0.06). Similarly, multiple bleeding episodes were associated with a higher proportion of low Apgar scores compared with single episodes, but without statistical significance (p = 0.07) (Table 3).
|
Characteristics |
First min APGAR scores <7 N=49 |
P-value |
|
Bleeding episode N (%) Single Multiple |
19 (38.8) 30 (61.2) |
0.07 |
|
Duration (days) N (%) 1– 2 > 2 |
18 (36.7) 31 (65.3) |
0.06 |
The mean birth weight of newborns was 2891 ± 539 g. While low birth weight was more common in the bleeding group, the difference was not statistically significant (Table 4). Overall, these findings suggest that first-trimester vaginal bleeding is associated with an increased risk of a low first-minute Apgar score at birth.
|
Neonatal birth weight (gm) |
Exposure group (first trimester bleeding) N = 218 |
Control group (without bleeding) N= 774 |
P-value |
|
LBW (<2500) N (%) |
48 (22) |
147 (19) |
0.07 |
|
Normal weight (2500-4000) N (%) |
159 (72.9) |
563 (72.7) |
0.4 |
|
Macrosomia (> 4000) N (%) |
11 (5.1) |
64 (8.3) | 0.2 |
Discussion
This study found a significant association between first-trimester vaginal bleeding and low one-minute Apgar scores. The Apgar score is a key indicator of neonatal health, and lower values often reflect perinatal distress and risk of complications such as hypoxic-ischemic encephalopathy and NICU admission [10,12].
One of the most significant findings in this study was Neonates born to mothers with first-trimester bleeding were more likely to have a one-minute Apgar <7 (22.5% vs. 6.2%, p = 0.02). This finding is consistent with previous research suggesting that early pregnancy bleeding may compromise fetal growth and lead to neonatal distress [5,6,15-17,24].
Karimi et al. reported in their meta-analysis that vaginal bleeding during pregnancy is a risk factor for adverse outcomes, including low Apgar scores and preterm birth [5]. Bever et al. found that first-trimester bleeding was linked to altered fetal growth patterns, which can contribute to neonatal distress and first minute low Apgar [6]. Some of these studies indicate a correlation between first-trimester bleeding and low Apgar scores at one and five minutes of birth [15-17, 24].
The underlying mechanism may involve placental dysfunction. Early bleeding may indicate subchorionic hematoma or implantation abnormalities, which can reduce placental efficiency and lead to fetal hypoxia. Gaillard et al. [1] reported that placental dysfunction adversely affects fetal growth and development, potentially manifesting as low Apgar scores. Maternal inflammation during early bleeding episodes may also negatively influence fetal development [15].
Although, some studies conversely have reported that first-trimester bleeding has no effect on newborn Apgar scores [18- 21], therefore, it seems that more studies are needed in this objective.
The absence of a significant difference in five-minute Apgar scores in our study the groups (bleeding: 8.7% vs. control: 6.7%, p = 0.6) suggests that prompt neonatal care and resuscitation may mitigate initial distress. This finding is consistent with Chen et al, who suggested that while low five-minute Apgar scores are predictive of long-term adverse outcomes, short-term resuscitation efforts often improve neonatal condition [11]. Current guidelines from the American Academy of Pediatrics recommend that neonates with low Apgar scores receive immediate and thorough evaluation to mitigate the risks associated with potential perinatal asphyxia [7].
Longer or recurrent bleeding episodes appeared to increase the risk of low Apgar scores, though not significantly. Chandrakala and Reshmi similarly noted that recurrent bleeding episodes often indicate placental dysfunction and can contribute to perinatal morbidity [24].
Although low birth weight was more common in the bleeding group, the difference was not statistically significant, differing from studies by Karimi et al, and Velez et al, possibly due to differences in population size and inclusion criteria [5,22]. The discrepancy may be attributed to variations in study populations, sample sizes, and differing criteria for defining low birth weight.
Conclusion
This study underscores the importance of vigilant prenatal monitoring in pregnancies affected by early first-trimester bleeding. Further investigations are required to identify predictors of adverse neonatal outcomes and to develop preventive measures that may enhance newborn health.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: The study was approved by the Institutional Ethics Committee and utilized data obtained from hospital archives.
Patient consent (participation and publication): Was obtained from all participants prior to completing the questionnaire, and participants were assured of confidentiality and anonymity.
Funding: The present study received no financial support.
Acknowledgements: The authors thank the residents of the Obstetrics and Pediatrics Departments at Shahid Sadoughi University of Medical Sciences, Yazd, Iran, for their assistance in data collection, and the Department of Statistics for support with data analysis.
Authors' contributions: LS Contributed to drafting the manuscript and critically revising its content, and approved the final version prior to submission. AJ Responsible for data acquisition, study conception and design, as well as data analysis and interpretation. All authors read and approved the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Not applicable.
Impact of Common Anticoagulants on Complete Blood Count Parameters Among Humans
Rawezh Q. Salih, Dahat A. Hussein, Sharaza Q. Omer, Shvan L. Ezzat, Ayman M. Mustafa, Hawnaz S....
Abstract
Introduction
Among the most frequently used anticoagulants in hematological testing are tetra-acetic acid (EDTA), sodium citrate, and sodium heparin. However, there is a noticeable gap in literature concerning the effects of these anticoagulants on hematological parameters specifically in humans. This study aims to assess the effectiveness of EDTA, sodium citrate, and sodium heparin for conducting complete blood count (CBC).
Methods
This cross-sectional study conducted at Smart Health Tower from January to April 2024 involved 250 participants who underwent CBC using K2EDTA, sodium citrate, and sodium heparin. The acquired data were analyzed using SPSS, with a significance level of p < 0.05, employing Intra-class correlation coefficient and one-way ANOVA to assess consistency and agreement among anticoagulants.
Results
A total of 250 participants, with 138(55.2%) males and 112(44.8%) females, underwent CBC testing with di potassium EDTA(K2EDTA), sodium citrate, and sodium heparin. Comparing K2EDTA with sodium heparin showed comparable values in 14 out of 23(60.87%) CBC parameters. Using K2EDTA as the standard, citrate showed perfect or substantial agreement in assessing 8 out of 23 CBC parameters (34.78%). Regarding the comparison of anticoagulants to K2EDTA to determine their agreement levels while sodium heparin was accurate and precise in 13(56.52%) parameters.
Conclusion
Citrate was found to be a less reliable anticoagulant for CBC estimation compared to K2EDTA, potentially leading to inaccurate readings. On the other hand, sodium heparin showed comparable performance to K2EDTA, making it a suitable alternative under specific conditions.
Introduction
The Complete Blood Count (CBC) is a widely requested blood test by clinicians, assessing the total quantities and characteristics of cellular constituents within the bloodstream. The CBC parameters include red blood cells (RBCs), white blood cells (WBCs), and platelets (PLTs). This comprehensive assessment includes determining the total and differential count of WBCs, also measuring RBC count, hemoglobin (HGB) levels, and hematocrit (HCT), as well as their indices such as mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), and red cell distribution width (RDW). Additionally, CBC evaluates platelet (PLT) count indices [1,2]. A CBC serves as an important diagnostic tool for assessing human health, detecting congenital abnormalities, and identifying functional changes due to various pathological factors [3]. Its findings can reveal various conditions such as infections with elevated WBC counts, leukemia with abnormal WBC counts, anemia with low HGB levels, and liver cirrhosis with reduced PLT counts. Recent studies suggest that specific combinations of CBC components, along with derived secondary results, can predict risks of different diseases like cardiovascular disease, cancer, type 2 diabetes, and metabolic syndrome [1,2].
It's widely recognized that collection and sampling of blood, laboratory techniques and storage conditions, and the choice of anticoagulant can substantially impact the outcomes derived from hematological analysis, especially CBC results [4]. Among the various anticoagulants used for both sample collection and routine laboratory analysis, the most commonly utilized ones in hematology are ethylene diamine tetra acetic acid (EDTA), citric acid salts, sodium and lithium oxalates, and heparin [5,6].
The National Committee for Clinical Laboratory Standards has suggested using EDTA for CBC due to its ability to preserve cell structure [7]. However, limited evidence exists on the effects of other anticoagulants on CBC parameters among animal species. Among humans, heparin is typically avoided for blood smears and WBC counts due to staining and clotting issues, respectively. Conversely, EDTA is considered unsuitable for erythrocyte osmotic fragility assessment and may cause cell damage if overused [8].
The majority of studies documented in existing literature have focused on evaluating the impacts of different anticoagulants on CBC results or its specific components across diverse animal species. The current study aims to estimate variations in CBC parameters using different anticoagulants, employing dipotassium EDTA(K2EDTA), sodium citrate, and sodium heparin, among humans to evaluate their effectiveness.
Methods
Study Design, population, and criteria
This cross-sectional laboratory-based study was conducted at Smart Health Tower from January to April 2024. Prior to participation, all individuals were thoroughly briefed about the study and required to provide informed written consent. The study included a total of 250 participants, all of whom underwent complete blood count tests utilizing K2EDTA, sodium citrate, and sodium heparin as anticoagulants. The study population consisted of patients attending Smart Health Tower, representing both genders without any gender bias. Inclusion was restricted to those who had visited the facility, while individuals or their guardians ( in case of minors) who declined to provide consent were excluded from the study.
Determination of the sample size
The effective sample size was determined using G*Power statistic 3.1.9.7, employing linear multiple regression as the statistical test with a two-tailed approach. With an effective sample size of 0.35, an α error probability of 0.01, and a statistical power of 0.99, along with a predictor value of 1, the minimum required sample size was 158. Therefore, a sample size of 250 was utilized for the comparison in CBC parameters between these three different anticoagulants.
Sample collection and statistical analysis
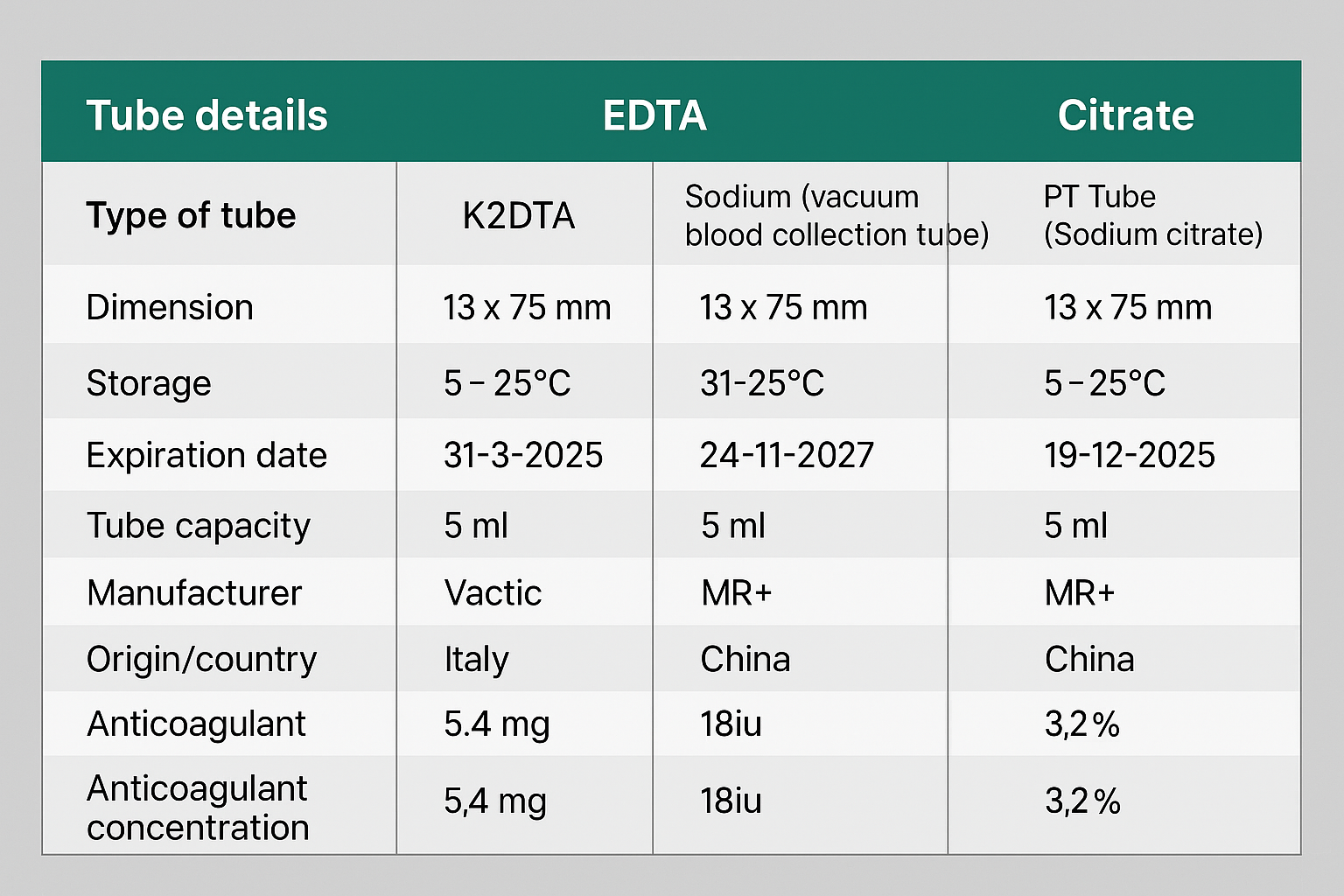
Trained health workers collected blood samples from participants using sterile syringes and needles, drawing 5 mL from either the median cubital or prominent forearm vein. The samples were distributed as follows: 1.8 mL into sodium citrate tubes and 1.6 mL into K2EDTA and sodium heparin tubes. After gentle mixing, complete blood counts (CBC) were analyzed with the Medonic M51 automated hematology analyzer within 3 to 6 hours post-collection. Tube characteristics are detailed in Table 1. Various hematological parameters were assessed, including WBC, percentages of neutrophils, lymphocytes, monocytes, eosinophils, basophils, as well as RBC, HCT, HGB, MCV, MCHC, RDW-SD, PLT, MPV, PDW, PCT, and PLCR. Participant demographics, such as age and gender, were also recorded. Data were initially processed in Microsoft Excel 2019 for accuracy and completeness before being transferred to SPSS version 25.0 and MedCalc version 20 for statistical analysis. Intra-class correlation coefficient (ICC) analysis was conducted to evaluate consistency among the three anticoagulants, with interpretations as follows: <0.50 for poor consistency, 0.50-0.75 for moderate, 0.75-0.90 for good, and >0.90 for excellent consistency. A p-value of <0.05 was considered significant. One-way ANOVA assessed variations in CBC parameters among samples collected in K2EDTA, sodium citrate, and sodium heparin tubes. Additionally, the concordance correlation coefficient (CCC) was used to evaluate agreement, with K2EDTA as the standard, and interpreted as follows: ≥0.99 for almost perfect agreement, 0.95-0.99 for significant agreement, 0.90-0.95 for moderate agreement, and <0.90 for poor agreement [9].
|
Tube details |
EDTA |
Heparin |
Citrate |
|
Type of tube |
K2EDTA |
Sodium (vacuum blood collection tube) |
PT Tube (Sodium citrate) |
|
Dimension |
13 x 75 mm |
13 x 75 mm |
13 x 75 mm |
|
Storage |
5- 25°C |
5-25°C |
5-25°C |
|
Expiration date |
31-3-2025 |
24-11-2027 |
19-12-2025 |
|
Tube capacity (volume) |
5 ml |
5 ml |
5ml |
|
Required volume |
1.5-2 ml |
1.5-2ml |
1.8ml |
|
Tube material |
Plastic |
glass |
glass |
|
Manufacturer |
Vacutest kima sri |
MR+ |
MR+ |
|
Origin/country |
Italy |
China |
China |
|
Anticoagulant concentration |
5.4 mg |
18iu |
3.2% |
Results
Among the 250 participants involved, 138 (55.2%) were male, and 112 (44.8%) were female. The participants had an average age of 41.20 ± 16.51 years (5-91). Consistency in CBC results using sodium heparin, K2EDTA, and sodium citrate indicated excellent consistency in the determination of WBC, %Neu, %Lymph, Neu, Lymph, RBC, HGB, HCT, MCV, MCH, MCHC, RDW-SD, MPV, PDW, and PLCR among these anticoagulants with ICC >0.90 (Table 2).
|
CBC parameters |
Intra-class correlation coefficient |
Confidence interval 95% |
|
|
Lower |
Upper |
||
|
WBC |
0.991 |
0.972 |
0.996 |
|
%Neu |
0.961 |
0.880 |
0.981 |
|
%Lymph |
0.987 |
0.984 |
0.990 |
|
%Mon |
0.494 |
0.038 |
0.717 |
|
%Eos |
0.869 |
0.838 |
0.895 |
|
%Bas |
0.733 |
0.636 |
0.801 |
|
Neu |
0.988 |
0.961 |
0.994 |
|
Lymph |
0.987 |
0.973 |
0.993 |
|
Mon |
0.612 |
0.120 |
0.802 |
|
Eos |
0.182 |
0.038 |
0.367 |
|
Bas |
0.803 |
0.719 |
0.858 |
|
RBC |
0.922 |
0.328 |
0.976 |
|
HGB |
0.923 |
0.321 |
0.976 |
|
HCT |
0.902 |
0.449 |
0.964 |
|
MCV |
0.998 |
0.994 |
0.999 |
|
MCH |
0.996 |
0.992 |
0.998 |
|
MCHC |
0.963 |
0.921 |
0.979 |
|
RDW-SD |
0.924 |
0.901 |
0.941 |
|
PLT |
0.536 |
0.276 |
0.689 |
|
MPV |
0.915 |
0.843 |
0.948 |
|
PDW |
0.921 |
0.880 |
0.945 |
|
PCT |
0.563 |
0.104 |
0.763 |
|
PLCR |
0.930 |
0.856 |
0.960 |
Regarding variation in CBC parameters using K2EDTA, sodium citrate, and sodium heparin, no statistically significant variation was found in the median %Lymph, Eos, MCV, and MCH among these three different anticoagulants (Table 3).
|
CBC parameters |
Sodium Heparin Median (Min-Max) |
Citrate |
K2EDTA |
P-value |
|
WBC |
7.54(2.52-26.26) |
7.21(2.26-23.85) |
7.51(2.54-26.10) |
0.046 |
|
%Neu |
61.15(37.2-92.6) |
56.70(37.60-90.90) |
56.30(30.70-91.80) |
<0.001 |
|
%Lymph |
33.2(3.8-50.30) |
33.55(4-50.50) |
33.45(3.90-52.60) |
0.718 |
|
%Mon |
1.9(0.0-12.20) |
6(0.20-13) |
6.45(0.80-14.10) |
<0.001 |
|
%Eos |
2.5(0.10-22.50) |
2.50(0.20-24.30) |
6.45(0.80-14.10) |
<0.001 |
|
%Bas |
0.65(0.20-3.10) |
0.50(0.10-2.20) |
0.50(0.10-1.50) |
<0.001 |
|
Neu |
4.92(0.94-24.31) |
4.40(0.99-21.68) |
4.65(0.78-23.96) |
0.015 |
|
Lymph |
2.41(0.39-5.76) |
2.31(0.37-5.40) |
2.49(0.45-5.99) |
0.049 |
|
Mon |
0.15(0.00-0.93) |
0.43(0.01-1.06) |
0.49(0.05-1.17) |
<0.001 |
|
Eos |
0.20(0.01-2.08) |
0.18(0.01-2.19) |
0.16(0.01-2.55) |
0.730 |
|
Bas |
0.05(0.01-0.23) |
0.04(0.01-0.17) |
0.04(0.01-0.13) |
<0.001 |
|
RBC |
5.12(2.47-7.65) |
4.62(2.21-6.35) |
5.13(2.48-7.05) |
<0.001 |
|
HGB |
14.2(6.90-21.30) |
12.6(6.20-16.9) |
14.10(6.90-18.20) |
<0.001 |
|
HCT |
43.15(21.4-64.8) |
38.90(19-51.20) |
43.45(3.72-54.60) |
<0.001 |
|
MCV |
85.45(56.90-108.5) |
85.15(56.8-108.4) |
85.9(57.3-108.6) |
0.534 |
|
MCH |
28.4(18.10-35.70) |
28.10(18-37.5) |
28.25(18.10-36.40) |
0.425 |
|
MCHC |
33(30.50-37.60) |
32.70(30.4-39.10) |
32.60(30-38.40) |
<0.001 |
|
RDW-SD |
43.5(34.70-63.10) |
43.40(34.60-64.00) |
44.10(35.30-82.20) |
0.016 |
|
PLT |
159(32-424) |
176(21-1584) |
250(86-482) |
<0.001 |
|
MPV |
9.30(6.90-12.10) |
8.80(4.40-11.80) |
9.10(7.10-13.00) |
<0.001 |
|
PDW |
11.85(7.30-21.10) |
11.10(2.60-20.00) |
11.60(8.10-23.60) |
<0.001 |
|
PCT |
0.15(0.03-0.34) |
0.15(0.02-0.70) |
0.22(0.09-0.37) |
<0.001 |
|
PLCR |
31.75(15.30-51.10) |
28.15(5.20-48.80) |
30.40(15.40-57.90) |
<0.001 |
Regarding variation in estimation of CBC parameters using the results of two anticoagulated blood such as K2EDTA-sodium citrate, K2EDTA-sodium heparin, sodium citrate-sodium heparin, the results of the comparison of K2EDTA-sodium citrate indicated comparable results in median %Neu, %Lymph, %Eos, Neu, Bas, MCV, MCH, and MCHC with a p-value of ≥0.05. Comparison of K2EDTA-sodium heparin results indicated comparable results in median WBC, %Lymph, %Eos, Neu, Lymph, RBC, HGB, HCT, MCV, MCH, RDW-SD, MPV, PDW, and PLCR with a p-value of ≥0.05. In comparing the results of CBC between sodium citrate-sodium heparin, the result indicated a nonsignificant difference in median WBC, %Lymph, %Eos, Lymph, MCV, MCH, RDW-SD, and PCT (Table 4).
|
CBC parameters |
Sodium Citrate |
Sodium Heparin |
P-value |
K2EDTA |
Sodium Heparin |
P-value |
K2EDTA |
Sodium Citrate |
P-value |
|
WBC |
7.21(2.26-23.85) |
7.54(2.52-26.26) |
0.121 |
7.51(2.54-26.10) |
7.54(2.52-26.26) |
0.941 |
7.51(2.54-26.10) |
7.21(2.26-23.85 |
0.05 |
|
%Neu |
56.70(37.60-90.90) |
61.15(37.2-92.6) |
<0.001 |
56.30(30.70-91.80) |
61.15(37.2-92.6) |
<0.001 |
56.30(30.70-91.80) |
56.70(37.60-90.90) |
0.788 |
|
%Lymph |
33.55(4-50.50) |
33.2(3.8-50.30) |
0.922 |
33.45(3.90-52.60) |
33.2(3.8-50.30) |
0.695 |
33.45(3.90-52.60) |
33.55(4-50.50) |
0.903 |
|
%Mon |
6(0.20-13) |
1.9(0.0-12.20) |
<0.001 |
6.45(0.80-14.10) |
1.9(0.0-12.20) |
<0.001 |
6.45(0.80-14.10) |
6(0.20-13) |
0.003 |
|
%Eos |
2.50(0.20-24.30) |
2.5(0.10-22.50) |
0.801 |
6.45(0.80-14.10) |
2.5(0.10-22.50) |
0.994 |
6.45(0.80-14.10) |
2.50(0.20-24.30) |
0.740 |
|
%Bas |
0.50(0.10-2.20) |
0.65(0.20-3.10) |
<0.001 |
0.50(0.10-1.50) |
0.65(0.20-3.10) |
<0.001 |
0.50(0.10-1.50) |
0.50(0.10-2.20) |
0.07 |
|
Neu |
4.92(0.94-24.31) |
4.92(0.94-24.31) |
0.011 |
4.65(0.78-23.96) |
4.92(0.94-24.31) |
0.288 |
4.65(0.78-23.96) |
4.92(0.94-24.31) |
0.345 |
|
Lymph |
2.41(0.39-5.76) |
2.41(0.39-5.76) |
0.282 |
2.49(0.45-5.99) |
2.41(0.39-5.76) |
0.633 |
2.49(0.45-5.99) |
2.41(0.39-5.76) |
0.040 |
|
Mon |
0.15(0.00-0.93) |
0.15(0.00-0.93) |
<0.001 |
0.49(0.05-1.17) |
0.15(0.00-0.93) |
<0.001 |
0.49(0.05-1.17) |
0.15(0.00-0.93) |
<0.001 |
|
Eos |
0.20(0.01-2.08) |
0.20(0.01-2.08) |
<0.001 |
0.16(0.01-2.55) |
0.20(0.01-2.08) |
<0.001 |
0.16(0.01-2.55) |
0.20(0.01-2.08) |
<0.001 |
|
Bas |
0.05(0.01-0.23) |
0.05(0.01-0.23) |
<0.001 |
0.04(0.01-0.13) |
0.05(0.01-0.23) |
<0.001 |
0.04(0.01-0.13) |
0.05(0.01-0.23) |
0.547 |
|
RBC |
5.12(2.47-7.65) |
5.12(2.47-7.65) |
<0.001 |
5.13(2.48-7.05) |
5.12(2.47-7.65) |
0.993 |
5.13(2.48-7.05) |
5.12(2.47-7.65) |
<0.001 |
|
HGB |
14.2(6.90-21.30) |
14.2(6.90-21.30) |
<0.001 |
14.10(6.90-18.20) |
14.2(6.90-21.30) |
0.816 |
14.10(6.90-18.20) |
14.2(6.90-21.30) |
<0.001 |
|
HCT |
43.15(21.4-64.8) |
43.15(21.4-64.8) |
<0.001 |
43.45(3.72-54.60) |
43.15(21.4-64.8) |
0.999 |
43.45(3.72-54.60) |
43.15(21.4-64.8) |
<0.001 |
|
MCV |
85.45(56.90-108.5) |
85.45(56.90-108.5) |
0.874 |
85.9(57.3-108.6) |
85.45(56.90-108.5) |
0.808 |
85.9(57.3-108.6) |
85.45(56.90-108.5) |
0.503 |
|
MCH |
28.4(18.10-35.70) |
28.4(18.10-35.70) |
0.391 |
28.25(18.10-36.40) |
28.4(18.10-35.70) |
0.773 |
28.25(18.10-36.40) |
28.4(18.10-35.70) |
0.807 |
|
MCHC |
33(30.50-37.60) |
33(30.50-37.60) |
0.009 |
32.60(30-38.40) |
33(30.50-37.60) |
<0.001 |
32.60(30-38.40) |
33(30.50-37.60) |
0.502 |
|
RDW-SD |
43.5(34.70-63.10) |
43.5(34.70-63.10) |
0.709 |
44.10(35.30-82.20) |
43.5(34.70-63.10) |
0.110 |
44.10(35.30-82.20) |
43.5(34.70-63.10) |
0.014 |
|
PLT |
159(32-424) |
159(32-424) |
0.001 |
250(86-482) |
159(32-424) |
<0.001 |
250(86-482) |
159(32-424) |
<0.001 |
|
MPV |
9.30(6.90-12.10) |
9.30(6.90-12.10) |
<0.001 |
9.10(7.10-13.00) |
9.30(6.90-12.10) |
0.211 |
9.10(7.10-13.00) |
9.30(6.90-12.10) |
<0.001 |
|
PDW |
11.85(7.30-21.10) |
11.85(7.30-21.10) |
<0.001 |
11.60(8.10-23.60) |
11.85(7.30-21.10) |
0.207 |
11.60(8.10-23.60) |
11.85(7.30-21.10) |
0.019 |
|
PCT |
0.15(0.03-0.34) |
0.15(0.03-0.34) |
0.022 |
0.22(0.09-0.37) |
0.15(0.03-0.34) |
<0.001 |
0.22(0.09-0.37) |
0.15(0.03-0.34) |
<0.001 |
|
PLCR |
31.75(15.30-51.10) |
31.75(15.30-51.10) |
<0.001 |
30.40(15.40-57.90) |
31.75(15.30-51.10) |
0.143 |
30.40(15.40-57.90) |
31.75(15.30-51.10) |
0.001 |
The agreement levels between different anticoagulants, using K2EDTA as the standard, were evaluated. Sodium citrate showed perfect agreement in assessing MCV and MCH (CCC = 0.990) but displayed significant agreement in determining WBC, %Neu, %Lymph, Neu, Lymph, and Eos (CCC between 0.95 and 0.99). Moderate agreement was observed in assessing MCHC (CCC = 0.929), while poor agreement was found in all other parameters with CCC<0.90. Similarly, sodium heparin demonstrated perfect agreement in determining MCV (CCC=0.994) and MCH (CCC=0.990), with substantial agreement in other parameters such as WBC, %Lymph, Neu, Lymph, RBC, and HGB (CCC between 0.95 and 0.99), but poor agreement in parameters with CCC<0.90. Regarding the comparison of K2EDTA and sodium citrate, citrate was highly precise and accurate in the estimation of WBC, %Neu, %Lymph, Neu, Lymph, Eos, MCV, MCH, and MCHC. While comparing sodium heparin to K2EDTA, it was highly precise in the estimation of WBC, %Neu, %Lymph, Neu, Lymph, Eos, RBC, HGB, HCT, MCV, MCH, MCHC, and PLCR (Table 5).
|
CBC parameters |
K2EDTA-Citrate |
Pearson ρ (precision) |
Accuracy |
K2EDTA-Sodium heparin |
Pearson ρ (precision) |
Accuracy |
|
WBC |
0.97(0.9571 -0.9718) |
0.988 |
0.977 |
0.985(0.981- 0.989) |
0.986 |
0.999 |
|
%Neu |
0.972(0.964-0.978) |
0.974 |
0.998 |
0.847(0.814-0.875) |
0.925 |
0.916 |
|
%Lymph |
0.984(0.980- 0.988) |
0.985 |
0.999 |
0.951(0.937- 0.961) |
0.954 |
0.997 |
|
%Mon |
0.663(0.593 - 0.723) |
0.705
|
0.941 |
0.157(0.111 -0.201) |
0.440 |
0.355 |
|
%Eos |
0.636(0.567 - 0.697) |
0.688 |
0.926 |
0.594(0.525 - 0.656) |
0.675 |
0.881 |
|
%Bas |
0.460(0.361 -0.548) |
0.480 |
0.958 |
0.305(0.209 - 0.396) |
0.371 |
0.822 |
|
Neu |
0.980(0.975 - 0.984) |
0.990 |
0.990 |
0.972(0.964 - 0.978) |
0.980 |
0.991 |
|
Lymph |
0.956(0.949 - 0.966) |
0.984 |
0.974 |
0.969(0.961 - 0.976) |
0.973 |
0.997 |
|
Mon |
0.733(0.675 - 0.782) |
0.795 |
0.922 |
0.209(0.157 - 0.261) |
0.500 |
0.419 |
|
Eos |
0.968(0.960 - 0.975) |
0.973 |
0.995 |
0.927(0.909 - 0.941) |
0.942 |
0.983 |
|
Bas |
0.565(0.477 - 0.642) |
0.576 |
0.990 |
0.458(0.371 -0.537) |
0.543 |
0.848 |
|
RBC |
0.723(0.691 - 0.764) |
0.983 |
0.742 |
0.973(0.966-0.979) |
0.974 |
0.999 |
|
HGB |
0.742(0.705 - 0.775) |
0.988 |
0.752 |
0.977(0.971 - 0.982) |
0.979 |
0.998 |
|
HCT |
0.670(0.620 - 0.714) |
0.911 |
0.735 |
0.907(0.882 - 0.926) |
0.909 |
0.998 |
|
MCV |
0.990(0.987 - 0.992) |
0.995 |
0.995 |
0.994(0.992 - 0.995) |
0.995 |
0.998 |
|
MCH |
0.990(0.987 - 0.992) |
0.991 |
0.998 |
0.990(0.987 - 0.992) |
0.992 |
0.998 |
|
MCHC |
0.929(0.910 - 0.944) |
0.933 |
0.995 |
0.874(0.844 - 0.898) |
0.932 |
0.937 |
|
RDW-SD |
0.721(0.661 - 0.772) |
0.762 |
0.946 |
0.738(0.681- 0.786) |
0.771 |
0.958 |
|
PLT |
0.313(0.236 - 0.386) |
0.470 |
0.668 |
0.290(0.235 - 0.343) |
0.648 |
0.448 |
|
MPV |
0.784(0.734 - 0.826) |
0.830 |
0.945 |
0.873(0.841 - 0.899) |
0.887 |
0.985 |
|
PDW |
0.790(0.740 - 0.832) |
0.815 |
0.970 |
0.819(0.774 - 0.856) |
0.832 |
0.983 |
|
PCT |
0.319(0.256 - 0.380) |
0.598 |
0.534 |
0.238(0.187- 0.289) |
0.585 |
0.408 |
|
PLCR |
0.833(0.794- 0.866) |
0.879 |
0.948 |
0.876(0.847- 0.901) |
0.901 |
0.973 |
Discussion
The choice of anticoagulants and storage time significantly affect blood sample analysis [10]. In a study by Akorsu et al. involving 55 healthy individuals, consistency in blood parameters across three anticoagulants was observed: K3EDTA, sodium citrate, and lithium heparin [3]. Similarly, a current study utilized K2EDTA, sodium citrate, and sodium heparin, finding excellent consistency in various blood parameters, with ICC values exceeding 0.90.
Regarding variation in CBC parameters using different anticoagulants, in a study which is conducted on 30 clinically healthy dogs from different breeds, no significant variation between sodium citrate and K3EDTA was found in 4 out of 8 CBC parameters (50%) including HGB, HCT, PLT, and PCT [11]. Similarly, in a study conducted on humans, in which variation in the estimation of CBC parameters was evaluated using three different anticoagulants, namely, K3EDTA, sodium citrate, and lithium heparin, no statistically significant difference was observed in 5 out of 14 CBC parameters (35.7%) including MCV, MCH, MCHC, %Lymph, and %Neu among the three anticoagulants examined [3]. In the present study, regarding variation in CBC parameters using K2EDTA, sodium citrate, and sodium heparin, no statistically significant variation was found in 4 out of 23 CBC parameters (17.40%) including %Lymph, Eos, MCV, and MCH among these three different anticoagulants. The significant variations observed in other CBC parameters underscore the need for careful consideration when selecting anticoagulants, particularly in clinical settings where precise and consistent CBC measurements are crucial for accurate diagnosis and monitoring of conditions [12].
In a study of 50 healthy dogs comparing EDTA and sodium citrate, no comparable results were found among 9 CBC parameters, suggesting citrate may lead to inaccurate results compared to EDTA [13]. Another study of 55 healthy individuals comparing heparin and citrate revealed significant differences in 5 out of 14 CBC parameters (35.71%), with the remaining parameters showing variations. Similar patterns were observed when comparing citrate to EDTA. Comparing heparin to K3EDTA showed significant variations in three parameters (21.43%) [3]. In the current study, comparing K2EDTA to sodium citrate showed similar results in 8 out of 23 CBC parameters (34.78%), while comparing K2EDTA to sodium heparin showed comparable values in 14 out of 23 CBC parameters (60.87%).
Comparing PLT results between K2EDTA and sodium citrate with sodium heparin, significantly lower PLT counts were found in the latter two in the current study, contradicting findings in existing genuine literature [14-16]. One study suggested that citrate's strong platelet activation in sick animals may lead to decreased PLT counts due to platelet clumping [17]. Additionally, lower HGB and HCT values were observed in citrated blood samples compared to EDTA, consistent with previous studies [3,11]. This discrepancy may be attributed to citrate's interference with HGB oxidation, resulting in higher HGB levels in EDTA samples.
The CBC is commonly conducted on venous blood specimens anticoagulated with EDTA. Among various EDTA subtypes, the dipotassium salt form, K2EDTA, is endorsed by the International Council for Standardization in Hematology as the preferred anticoagulant for blood cell enumeration and sizing [7]. The study evaluated agreement levels between different anticoagulants, using K2EDTA as the standard. Sodium citrate showed substantial agreement in 8 out of 23 CBC parameters (34.78%), including MCV, MCH, WBC, %Neu, %Lymph, Neu, Lymph, and Eos. Similarly, sodium heparin demonstrated substantial agreement in determining MCV, MCH, WBC, %Lymph, Neu, Lymph, RBC, and HGB. These findings align with previous literature, which indicated substantial agreement with heparin in assessing 4 out of 14 CBC parameters (28.57%), including RBC, HGB, HCT, and MCH [3].
Conclusion
Citrate was found to be a less reliable anticoagulant for CBC estimation compared to K2EDTA, potentially leading to inaccurate readings. On the other hand, sodium heparin showed comparable performance to K2EDTA, making it a suitable alternative under specific conditions.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: The study was approved by the Institutional Ethics Committee and utilized data obtained from hospital archives Ethical Approval for this study was obtained from the Ksciens ethical committee (Approval Number 43. 2025).
Patient consent (participation and publication): Written informed consent was obtained from all patients (or their legal guardians, where applicable) for participation in the study and for the publication of all associated clinical information and images.
Source of Funding: Star Lab Company.
Role of Funder: The funder remained independent, refraining from involvement in data collection, analysis, or result formulation, ensuring unbiased research free from external influence.
Acknowledgements: Not applicable.
Authors' contributions: RQS and SQO were major contributors to the conception of the study, as well as to the literature search for related studies. DAH and AMM were involved in the literature review and the writing of the manuscript. SLE, HAY, HSA and MTT were involved in the literature review, the design of the study, the critical revision of the manuscript, and the processing of the tables. QOS and AMM confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Exploring the Efficacy of Once and Twice Weekly Thyroxine Dosing: A Promising Approach for Hypothyroidism Management
Abdulwahid M. Salih, Aso S. Muhialdeen, Mohsin M. Ahmed, Hardi M. Zahir, Yadgar A. Saeed,...
Abstract
Introduction
Hypothyroidism is a common endocrine disorder, in which the management involves daily intake of thyroxine. However, adherence to a daily medication regimen poses a substantial challenge for many patients. The current study aims to assess the efficacy of once and twice-weekly thyroxine regimen for the management of hypothyroidism.
Methods
This was a single-center cohort study involving hypothyroid patients that was conducted over three years. In this study, standard daily, once and twice-weekly thyroxine dosing regimens were used to treat the patients. The effectiveness of the three dosage regimens was ascertained by whether the patients achieved a euthyroid state after six months of therapy.
Results
In total, 328 hypothyroid cases due to thyroiditis were included in this study. The average age of the cases was 42.7 years (14-92). Before thyroxine therapy, in the standard daily regimen group, the median level of TSH was 15.4 μIU/mL (IQR 23.1), in the once-weekly regimen group, the level was 9.2 μIU/mL (IQR 6.8), and in the twice-weekly regimen group, the level was 9.1 μIU/mL (IQR 7.3). After thyroxine intake and upon follow-up after 6 months, the TSH level decreased to 4.0 μIU/mL (IQR 3.7), 4.5 μIU/mL (IQR 4.9) and 4.2 μIU/mL (IQR 5.1) in the standard daily, once- and twice-weekly regimen groups, respectively.
Conclusion
Once and twice weekly thyroxine shows promise as strategies for managing hypothyroidism. However, variability in patients in response to weekly thyroxine needs to be taken into account.
Introduction
Hypothyroidism is a condition characterized by the inadequate production of thyroid hormones and represents a common endocrine disorder affecting a significant proportion of the global population [1]. The standard management of this condition typically involves daily administration of synthetic thyroxine to normalize thyroid hormone levels, thereby alleviating associated symptoms and metabolic disturbances. Daily thyroxine therapy regimen is well-established in clinical practice, with its efficacy extensively documented in the medical literature [1,2]. However, adherence to a daily medication regimen can be challenging for many patients. Factors such as the requirement to take the medication on an empty stomach, the necessary waiting period before the next meals or beverages, and potential drug interactions may contribute to inconsistent compliance. Suboptimal adherence to daily thyroxine therapy can significantly impair the management of hypothyroidism, resulting in inadequate control of thyroid function and poorer patient outcomes [3].
Recently, non-daily alternative dosing schedules, specifically once or twice-weekly thyroxine administration, have gained attention as potential strategies to improve adherence. Nevertheless, studies evaluating the efficacy of these regimens remain limited, and current evidence is insufficient to support changes in clinical practice. The current study aims to assess the efficacy of once and twice-weekly thyroxine dosing compared to standard daily administration for the management of hypothyroidism.
Methods
Study design
This was a single-center cohort study conducted at the head and neck center of Smart Health Tower, Sulaimani, Iraq, from January 2020 to January 2023. It involved the retrospective analysis of a series of hypothyroid cases to evaluate the efficacy of once and twice-weekly thyroxine regimens compared to standard daily dose. Written informed consent was obtained from the patients and the family of the patients.
Participation
Participants were individuals who visited the head and neck center for the treatment of hypothyroidism. Patients who had undergone thyroidectomy were excluded from the study to avoid confounding effects on thyroid function.
Treatment plan
Patients were assigned to one of three treatment groups based on the prescribed thyroxine regimen: daily, once-weekly, or twice-weekly. According to thyroid function tests and body weight, different daily and weekly doses were prescribed to the patients to reach euthyroidism, including 25, 50, 75, or 100 μg per day, or 100, 200, or 300 μg per week. Patients were followed for six months, and treatment effectiveness was determined by whether they achieved a euthyroid state, defined as a thyroid stimulating hormone (TSH) level between 0.4 and 4.2 μIU/mL.
Data collection and analysis
Data were extracted from the center’s medical database and included patient’s age, gender, marital status, occupation, chief complaint, thyroxine dosage, TSH pre-treatment, and TSH six months following therapy. All data were entered into an Excel spreadsheet (2016). Statistical analyses were performed using statistical package for social sciences (SPSS) version 25. Descriptive statistics were presented as means or medians (for non-normally distributed data), counts, and percentages. Univariate analyses were conducted to compare baseline characteristics (including age, gender, marital status, occupation, and chief complaint) and treatment outcomes between groups. Additionally, multivariate logistic regression analysis was performed to identify independent predictors of achieving normal TSH (0.4–4.2 μIU/mL) at six months. A p-value of less than 0.05 was considered statistically significant.
Results
In total, 328 hypothyroid cases due to thyroiditis were included in this study. The mean age of the cases was 42.7 years, ranging from 14 to 92 years. The majority of the cases were female, accounting for 303 individuals (92.4%). The most common presentation in all groups was weakness in 146 (44.5%) cases, followed by incidental finding in 81 (24.7%), and neck pain in 37 (11.3%). Overall, the first TSH mean (before therapy) was 20.8, while the second TSH mean (after treatment) was 5.1 (Table 1).
|
Characteristics |
Frequency/Mean |
Percentage/SD |
|
Age |
42.7 |
13.1 |
|
First TSH |
20.8 |
25.1 |
|
Second TSH |
5.1 |
4.2 |
|
Sex |
||
|
Male Female |
25 303 |
7.6 92.4 |
|
Occupation |
||
|
Employed Jobless Lawyer Student Teacher Others |
18 247 3 21 16 23 |
5.5 75.3 0.9 6.4 4.9 7 |
|
Marital Status |
||
|
Married Single |
283 43 2 |
86.3 13.1 0.6 |
|
Chief Complain |
||
|
Abnormal Menstruation Cold Intolerance Hair Loss Incidental Neck Pain Neck Swelling Neck Weakness Others |
19 6 5 81 37 18 146 16 |
5.8 1.8 1.5 24.7 11.3 5.5 44.5 4.6 |
|
Treatment |
||
|
1/week 2/week Daily |
100 119 109 |
30.5 36.3 33.2 |
| TSH: Thyroid stimulating hormone, SD: Standard deviation | ||
The median age was similar across the three groups, with 42.0 years (IQR 13.5) in the once-weekly group, 40.0 years (IQR 16.0) in the twice-weekly group, and 43.0 years (IQR 23.0) in the daily group (p = 0.061). Females predominated in all groups, accounting for 98% of the once-weekly group, 97.5% of the twice-weekly group, and 81.7% of the daily group, with a statistically significant difference (p < 0.001). Regarding occupation, the majority were jobless across all groups, but the daily group had a higher proportion of employed participants (9.2%) compared with the weekly groups (p < 0.001) (Table 2).
|
Variables |
Treatment |
P-value | ||
|
1/week (n=100) |
2/week (n=119) |
Daily (n=109) |
||
|
Age, median (IQR) |
42.0 (13.5) |
40.0 (16.0) |
43.0 (23.0) |
0.061 |
|
Sex |
|
|
|
<0.001
|
|
Female |
98 (98.0%) |
116 (97.5%) |
89 (81.7%) |
|
|
Male |
2 (2.0%) |
3 (2.5%) |
20 (18.3%) |
|
|
Marital Status |
|
|
|
|
|
Married |
88 (88.0%) |
95 (79.8%) |
100(91.7%) |
0.012 |
|
Single |
10 (10.0%) |
24 (20.2%) |
9 (8.3%) |
|
|
Widow |
2 (2.0%) |
0 (0.0%) |
0 (0.0%) |
|
|
Chief Complain |
|
|
|
|
|
Abnormal Menstruation |
7 (7.0%) |
8 (6.7%) |
4 (3.7%) |
<0.001 |
|
Cold Intolerance |
0 (0.0%) |
4 (3.4%) |
2 (1.8%) |
|
|
Hair Loss |
2 (2.0%) |
1 (0.8%) |
2 (1.8%) |
|
|
Incidental |
5 (5.0%) |
6 (5.0%) |
70 (64.2%) |
|
|
Neck Pain |
15 (15.0%) |
17 (14.3%) |
5 (4.6%) |
|
|
Neck Swelling |
6 (6.0%) |
9 (7.6%) |
3 (2.8%) |
|
|
Neck Weakness |
59 (59.0%) |
69 (58.0%) |
18 (16.5%) |
|
|
Others |
6 (6.0%) |
5 (4.2%) |
5 (4.6%) |
|
|
Occupation |
|
|
|
|
|
Employed |
2 (2.0%) |
6 (5.0%) |
10 (9.2%) |
<0.001 |
|
Jobless |
79 (79%) |
92 (77.3%) |
76 (69.7%) |
|
|
Lawyer |
2 (2.0%) |
1 (0.8%) |
0 (0.0%) |
|
|
Student |
8 (8.0%) |
10 (8.4%) |
3 (2.8%) |
|
|
Teacher |
7 (7.0%) |
7 (5.9%) |
2 (1.8%) |
|
|
Others |
2 (2.0%) |
3 (2.5%) |
18 (16.5%) |
|
| IQR: Interquartile range | ||||
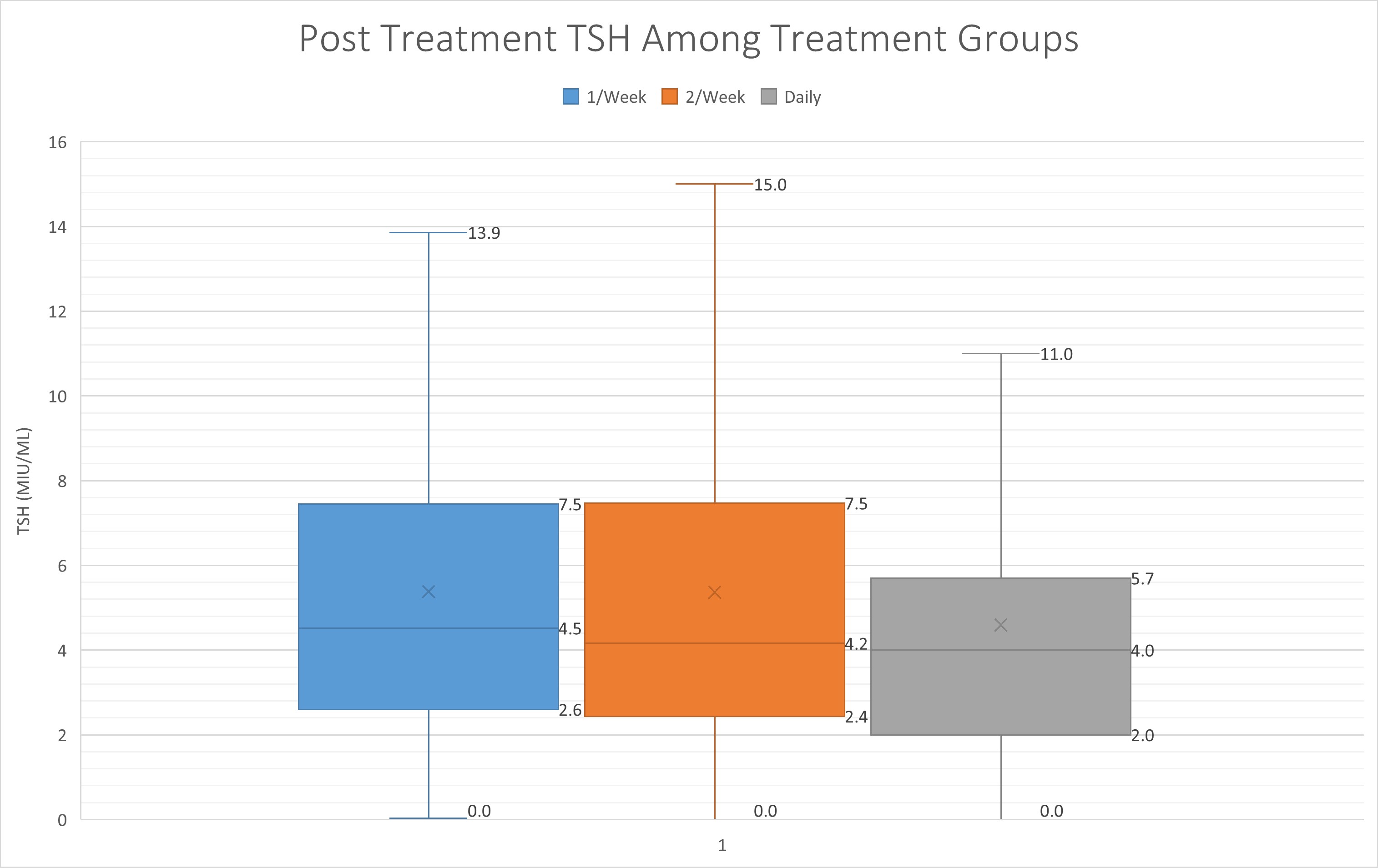
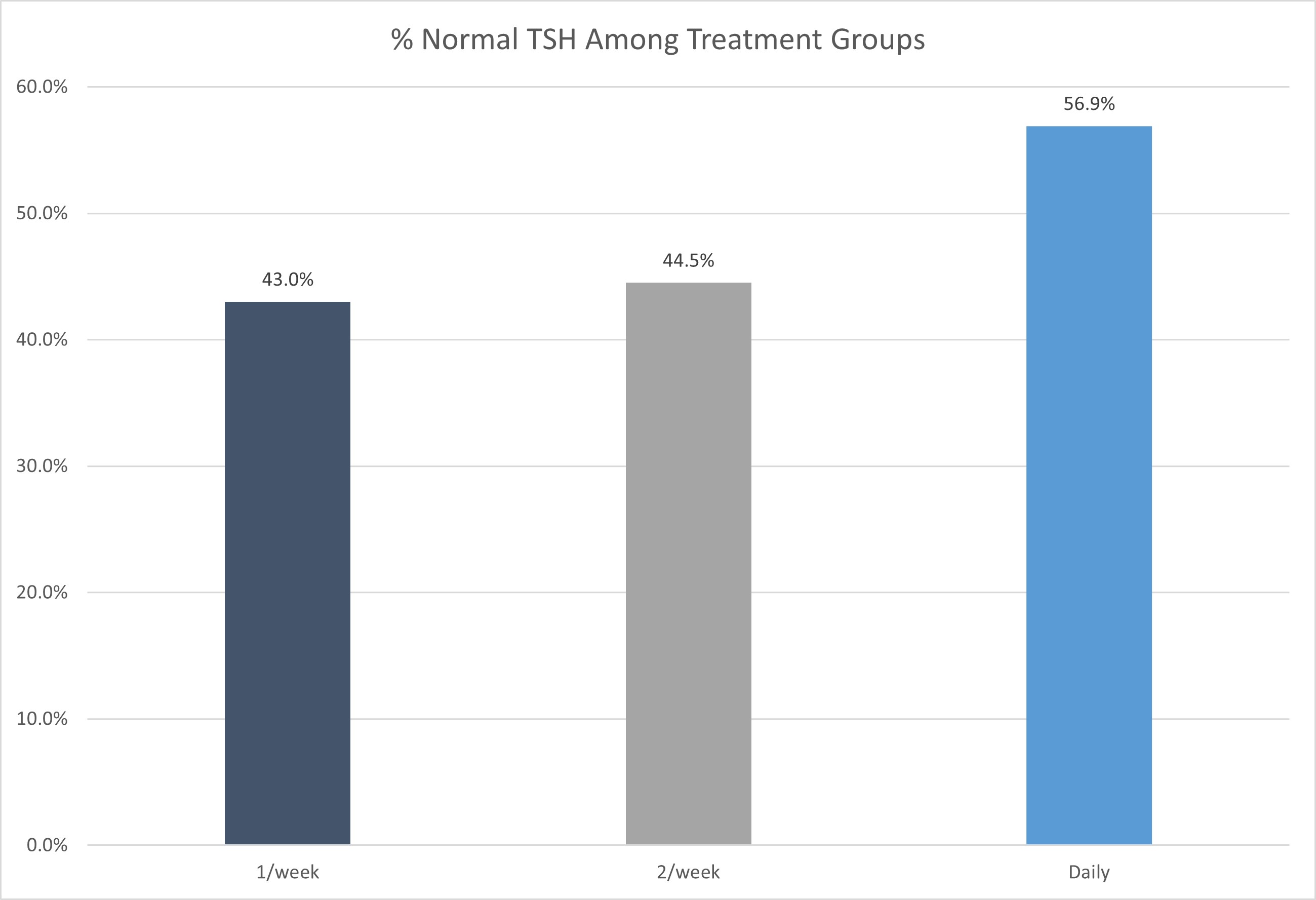
Before thyroxine therapy, in the once-weekly regimen group, the median value of TSH was 9.2 μIU/mL (IQR 6.8 μIU/mL), in the twice-weekly regimen group the level was 9.1 μIU/mL (IQR 7.3 μIU/mL), and in the standard daily dose group the median level was 15.4 (IQR 23.1 μIU/mL). After thyroxine intake and upon follow-up after six months, the TSH level decreased to 4.0 μIU/mL (IQR 3.7 μIU/mL), 4.2 μIU/mL (IQR 5.1 μIU/mL) and 4.5 μIU/mL (IQR 4.9 μIU/mL) in the standard daily, once- and twice-weekly regimen group, respectively (Figure 1) (Table 3). The outcome was statistically insignificant between groups. After six months of follow-up, 56.9% of patients in the daily regimen group, 43.0% in the once-weekly group, and 44.5% in the twice-weekly group achieved TSH levels within the normal reference range (Figure 2).
|
Variables |
Treatment |
P-value | ||
|
1/week (n=100) |
2/week (n=119) |
Daily (n=109) |
||
|
First TSH, median (IQR) |
9.2 (6.8) |
9.1 (7.3) |
15.4 (23.1) |
<0.001 |
|
Second TSH, median (IQR) |
4.5 (4.9) |
4.2 (5.1) |
4.0 (3.7) |
0.349 |
|
TSH Range |
|
|
|
|
|
<0.4 |
6 (6.0%) |
8 (6.7%) |
5 (4.6%) |
0.276 |
|
0.4 - 4.2 |
43 (43.0%) |
53 (44.5%) |
62 (56.9%) |
|
|
>4.2 |
51 (51.0%) |
58 (48.7%) |
42 (38.5%) |
|
| TSH: Thyroid stimulating hormone, IQR: Interquartile range | ||||
Compared to the daily treatment group, patients in the once-weekly group had an odds ratio (OR) of 0.659 (95% CI: 0.310–1.398, p = 0.277), and those in the twice-weekly group had an OR of 0.763 (95% CI: 0.363–1.602, p = 0.474), indicating no statistically significant difference in achieving normal TSH between the treatment regimens. Gender was not a significant predictor; males had an OR of 1.845 (95% CI: 0.439–7.751, p =0.403) compared to females. Age and baseline TSH similarly did not significantly influence the likelihood of achieving normal TSH (OR = 0.997, p = 0.801 and OR = 0.995, p = 0.317, respectively) (Table 4).
|
Parameters |
B (S.E) |
Exp (B) (OR) |
95% CI |
P-value |
|
Treatment Group Daily* 1/week 2/week |
-0.418 (0.384) -0.271 (0.379) |
1 0.659 0.763 |
0.310-1.398 0.363-1.602 |
0.277 0.474 |
|
Age (Years) |
-0.003 (0.010) |
0.997 |
0.978-1.017 |
0.801 |
|
Gender Female* Male |
0.612 (0.732) |
1 1.845 |
0.439-7.751 |
0.403 |
|
First TSH |
-0.005 (0.005) |
0.995 |
0.985-1.005 |
0.317 |
|
Occupation Employed* Jobless Lawyer Student Teacher Others |
0.497 (0.568) 0.201 (1.367) 0.196 (0.795) 0.267 (0.758) -0.138 (0.788) |
1 1.643 1.223 1.217 1.306 0.871 |
0.540-5.002 0.084-17.827 0.256-5.780 0.296-5.770 0.186-4.084 |
0.382 0.883 0.805 0.724 0.861 |
|
Marital Status Married Single Widow |
-0.249 (0.434) 0.104 (1.496) |
1 0.779 1.109 |
0.333-1.825 0.059-20.814 |
0.566 0.945 |
Discussion
Hypothyroidism can often occur as a result of thyroiditis or thyroidectomy to remove part of or the entire thyroid tissue [4-6]. The standard treatment for hypothyroidism involves daily administration of thyroxine, a synthetic form of the thyroid hormone. However, emerging evidence suggests that a weekly thyroxine regimen may offer several advantages in terms of patient convenience, treatment adherence, and potentially improved clinical outcomes [7]. Noncompliance with thyroxine is the most prevalent cause of poor hypothyroidism management, which is linked to the discomfort of taking the drug when fasting, waiting 60 minutes for the next meal or beverage, and avoiding other medications that may interfere with thyroxine absorption [8]. The feasibility of weekly thyroxine dosing lies in the pharmacokinetics of levothyroxine. It has a long half-life, with stable levels maintained in the bloodstream for several weeks. Additionally, the availability of thyroxine formulations that allow for extended release further supports the feasibility of weekly dosing [9].
One of the primary advantages of weekly thyroxine is the potential for enhanced patient convenience and improved treatment adherence. Daily medication regimens can be challenging for patients to adhere to consistently, leading to suboptimal management of hypothyroidism. Weekly thyroxine simplifies the dosing schedule, reducing the burden of daily medication intake [10]. Studies have shown that non-adherence to daily thyroxine therapy is common and can result in inadequate control of thyroid function. By reducing the frequency of dosing, weekly thyroxine may address the issue of non-adherence and improve the effectiveness of treatment in achieving target thyroid hormone levels and overall clinical outcomes [11].
There have been very few studies comparing daily versus weekly treatment of thyroxine in hypothyroid patients globally. Therefore, there is a crucial need to expand the current body of litrerature on this treatment strategy. According to one study, in today's world of busy lifestyles, when it is difficult for patients to take thyroxine on a strict schedule, once-weekly thyroxine administration gives an alternate option for treating hypothyroidism, particularly in patients whose compliance is a big concern [12]. Bornschein et al. found comparable results between daily and weekly dosing, with no hyperthyroidism in patients with weekly regimens [10]. Rangan et al. demonstrated in a case series of two patients that once-weekly thyroxine is an alternate treatment approach for noncompliant patients and remarked that once-weekly thyroxine is a safe treatment regimen [11]. Another study found that when compared to a daily dosage of thyroxine, once weekly thyroxine is an effective and safe long-term treatment option for individuals with poor management of hypothyroidism or apparent thyroxine resistance. With directly monitored once-weekly thyroxine, approximately three-quarters of such individuals are able to achieve TSH normalization [8]. According to Chiu et al., weekly thyroxine administration results in less suppression and greater overall TSH levels while keeping below the normal reference range as specified by worldwide treatment recommendations. It may be a viable option for hypothyroid individuals, especially if compliance is an issue [13].
Concerns have also been expressed that taking a big dose of once-weekly thyroxine may result in increased supraphysiologic levels of thyroxine in the first few days, which may have an unfavorable effect on cardiovascular function. It is also believed that by the sixth day of thyroxine administration, the efficacy of once-weekly thyroxine would have worn off, resulting in the recurrence of hypothyroid symptoms and swings in TSH levels [14]. Grebe et al. showed that, while weekly treatment was well tolerated, thyroid function tests showed significant hypothyroidism with an increase in TSH and a decrease in thyroxine before the next weekly prescription [15]. A meta-analysis of four trials including 294 participants evaluated the effect of once weekly thyroxine on TSH after 6 weeks of treatment. After 6 weeks of therapy, once weekly thyroxine patients had considerably greater serum TSH than standard daily thyroxine patients [14]. The results of the current study showed that once weekly and twice weekly thyroxine were almost equally effective as standard daily thyroxine in achieving and maintaining normal thyroid function. There were no significant differences between the three treatment groups.
This study has several limitations, including relatively small group sizes, a single-center design, and a short follow-up period of six months. Despite these limitations, the findings provide supportive evidence that may have implications for current clinical practice regarding thyroxine administration. Future research involving larger, multicenter cohorts with longer follow-up periods is warranted to validate and expand upon these results.
Conclusion
The comparison of once-daily, once-weekly, and twice-weekly thyroxine administration highlights the potential benefits of less frequent dosing for hypothyroidism management. While all regimens appear to maintain comparable efficacy and safety, the convenience and potential for improved adherence associated with less frequent dosing regimens are notable. However, patient response to weekly thyroxine can vary, and some individuals may require more frequent administration or may not be suitable candidates for weekly therapy. Additionally, infrequent dosing carries the risk of missed doses, which could compromise treatment effectiveness. Therefore, close monitoring of thyroid function and individualized dose adjustments are essential to optimize outcomes. Long-term studies are needed to evaluate the sustained efficacy, safety, and patient satisfaction with weekly thyroxine regimens. Further research comparing different thyroxine formulations for weekly dosing and assessing their impact on specific populations, such as pregnant women or patients with comorbidities, would be valuable.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Ethical approval for this study (Ethical Committee No: 95) was provided by the Ethical Committee of School of Medicine-University of Sulaimani.
Patient consent (participation and publication): Written informed consent was obtained from all patients and/or their legal guardians for participation in this study and for the publication of any accompanying images, clinical information, and other data included in the manuscript.
Source of Funding: Smart Health Tower.
Role of Funder: The funder remained independent, refraining from involvement in data collection, analysis, or result formulation, ensuring unbiased research free from external influence.
Acknowledgements: None to be declared.
Authors' contributions: AMS and SFA were major contributors to the conception of the study, as well as to the literature search for related studies. MNH, SHH and HAH were involved in the literature review and the writing of the manuscript. ASM, MMA, HMZ, YAS, SHA, HOB, MAA and SHA were involved in the literature review, the design of the study, the critical revision of the manuscript, and the processing of the tables. AMS and MNH confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Suffering of Patients with Neurogenic Thoracic Outlet Syndrome (TOS); The First Qualitative study in TOS
Fahmi H. Kakamad, Shvan H. Mohammed, Berun A. Abdalla, Saywan K. Asaad, Abdullah K. Ghafour,...
Abstract
Background
Diagnosis of neurogenic thoracic outlet syndrome (nTOS) is hindered by symptom overlap with cervical radiculopathy, carpal tunnel syndrome, or psychosomatic disorders. This challenge is further compounded by often normal imaging and electrodiagnostic findings, resulting in prolonged diagnostic suffering encompassing emotional, financial, and social burdens.
Objectives
This first qualitative study explores narratives from 25 diagnosed women initially prescribed physiotherapy: (1) identify key themes related to diagnostic challenges; (2) examine the psychological and emotional impact of diagnostic delay; and (3) propose a patient-centered diagnostic framework.
Methods
Qualitative descriptive design using semi-structured interviews (20-30 minutes) with purposive sampling from a TOS clinic (inclusion: confirmed nTOS, >1-year symptoms, no prior surgery; Data saturation was achieved after 22 interviews). Braun and Clarke’s reflexive thematic analysis generated 1,247 inductive codes, which were organized into four themes and twelve subthemes. NVivo software was used for data management. Member checking was conducted, and reporting followed COREQ guidelines. Ethical approval was obtained, and participant anonymity was preserved through pseudonyms.
Results
Four overarching themes emerged: (1) Fragmented Diagnostic Odyssey, characterized by multiple referrals (mean six clinicians per patient) and substantial out-of-pocket costs (USD 1,000–1,500); (2) Cascade of Misdiagnoses, including somatic mimics, invasive investigations, and prolonged incorrect treatment; (3) Social and Familial Invalidation, involving medical dismissal and pressure toward psychiatric explanations; and (4) Profound Emotional Suffering, with isolation and hopelessness identified in 84% of transcripts. A conceptual model was developed to illustrate the cumulative diagnostic journey.
Conclusion
Neurogenic thoracic outlet syndrome is associated with multilayered diagnostic and social invalidation consistent with the stigma of invisible illness. Improving outcomes requires enhanced clinician awareness of nTOS-specific red flags, validation of patient narratives, and multidisciplinary diagnostic pathways to reduce delays, prevent iatrogenic harm, and alleviate psychological distress.
Introduction
Neurogenic thoracic outlet syndrome (nTOS) is characterized by compression of the brachial plexus as it traverses the thoracic outlet, causing upper extremity pain, numbness, paresthesia, and variable degrees of weakness. The condition predominantly affects young women, often triggered by repetitive overhead activities, postural strain, or antecedent trauma. Despite its clinical relevance, nTOS remains one of the most diagnostically challenging peripheral nerve compression syndromes due to its heterogeneous and fluctuating presentation [1,2].
Clinical recognition is often delayed, as symptoms overlap substantially with more prevalent conditions such as cervical radiculopathy, carpal tunnel syndrome, peripheral neuropathies, and functional or psychosomatic disorders. The absence of definitive radiological or electrodiagnostic findings in many patients further compounds diagnostic uncertainty, contributing to prolonged diagnostic latency that may span several years [1,2]. Consequently, patients frequently undergo multiple inconclusive investigations and consultations before receiving an accurate diagnosis [1,2].
Beyond physical morbidity, individuals with nTOS experience a form of diagnostic suffering, encompassing emotional distress, financial strain, social disruption, and erosion of trust in healthcare systems. Recurrent symptom invalidation and misattribution may intensify anxiety, frustration, and feelings of marginalization. This experience closely parallels Bury’s concept of biographical disruption in chronic illness, whereby delayed or contested diagnoses fracture personal identity, future planning, and the patient–clinician relationship [3,4].
The present study seeks to illuminate these underexplored qualitative dimensions through in-depth narratives from 25 diagnosed women, all of whom were initially prescribed physiotherapy as first-line management. The study objectives are threefold: (1) to identify recurring themes related to diagnostic challenges and healthcare encounters; (2) to explore the psychological and emotional impact of prolonged diagnostic trajectories; and (3) to propose a patient-centered diagnostic framework that integrates biomedical assessment with experiential and psychosocial dimensions.
Methods
Study design
A qualitative descriptive study design with interpretive orientation was employed using semi-structured conversations to explore participants lived experiences of neurogenic thoracic outlet syndrome, this approach was selected to capture rich, first-person accounts while remaining close to participants’ language and meanings. The study was conducted and reported in accordance with the Consolidated Criteria for Reporting Qualitative Research (COREQ) checklist [5].
Participants and sampling
Purposive sampling recruited 25 female patients from a TOS specialty clinic. Inclusion: confirmed nTOS diagnosis, physiotherapy as initial treatment, symptom duration >1 year. Exclusion: surgical intervention prior to study. Saturation achieved after 22 interviews, with 3 additional for confirmation. Inclusion criteria were: (1) a confirmed diagnosis of neurogenic thoracic outlet syndrome, (2) initiation of physiotherapy as first-line management, and (3) symptom duration exceeding one year. Patients who had undergone surgical intervention prior to study enrollment were excluded. Data saturation was reached after 22 interviews, with three additional interviews conducted to confirm thematic completeness and stability.
Data collection
One-on-one conversations (20–30 minutes) in a private clinic room to ensure confidentiality and participant comfort A semi-structured interview guide was used to explore diagnostic trajectories, experiences of misdiagnosis, emotional and psychological impact, and social responses to symptoms. Sample prompts included: “Can you walk me through your journey to receiving a diagnosis?” and “How did family members or healthcare providers respond to your symptoms?” (Table 1). All interview were audio-recorded with participants’ consent and transcribed verbatim, yielding approximately 450 pages of textual data.
|
Interview Guide Domains |
Sample Questions |
|
Diagnostic Journey |
"What tests did you undergo? How many specialists?" |
|
Misdiagnoses & Treatments |
"What alternative diagnoses were suggested?" |
|
Emotional/Social Impact |
"How did this affect your relationships or self-view?" |
|
Validation & Recovery |
"What changed after nTOS diagnosis?" |
Data analysis
Braun and Clarke's (2006) reflexive thematic analysis in six phases (6):
- Familiarization: Repeated, immersive reading of transcripts to gain an overall understanding of the data.
- Coding: Inductive line-by-line coding was performed, generating 1,247 initial codes.
- Theme Generation: Collating into 4 main themes, 12 subthemes.
- Review: Preliminary themes were reviewed and refined through member checking with ten participants.
- Definition: Themes were clearly defined, refined, and supported by illustrative patient quotations.
- Reporting: Final themes were synthesized and presented using verbatim extracts to preserve participants’ voices.
NVivo 14 used for management. Methodological rigor and trustworthiness were enhanced through maintenance of an audit trail, thick description, reflexive journaling, and transparency regarding researcher positioning; the researchers declared no personal or clinical conflicts of interest related to thoracic outlet syndrome.
Ethical considerations
The study was conducted in accordance with ethical principles for human research. Written informed consent was obtained from all participants prior to participation. Pseudonyms were assigned to protect confidentiality. Participants were provided with debriefing following the conversations and offered referral resources for psychological or clinical support if needed.
Results
Analysis of interviews with 25 participants (coded P1–P25) yielded four overarching themes, each comprising related subthemes supported by illustrative verbatim quotations.
Theme 1: Fragmented Diagnostic Odyssey
Participants described a prolonged and exhausting diagnostic trajectory characterized by repeated referrals and inconclusive investigations across multiple medical specialties. On average, participants reported consulting approximately six physicians prior to receiving a diagnosis of neurogenic thoracic outlet syndrome.
Subtheme 1.1: Endless Investigations
"I have done hundreds of investigations; each doctor tells me a different diagnosis." (P7) "I cannot count how many doctors I visited; no one told me you have nTOS." (P14) These accounts highlight a cycle of uncertainty in which repeated testing failed to yield diagnostic closure.
Subtheme 1.2: Financial and Time Toll
The diagnostic delay was accompanied by substantial financial and temporal burden. Participants reported out-of-pocket expenditures ranging from approximately USD 1,000 to 1,500, in addition to lost workdays and prolonged functional impairment.
Theme 2: Cascade of Misdiagnoses
Symptom overlap with other neurological, musculoskeletal, and systemic conditions frequently resulted in misdiagnosis and inappropriate interventions.
Subtheme 2.1: Somatic Mimics: Several participants underwent unnecessary or unrelated treatments based on incorrect attribution of symptoms:
"I extracted three teeth; they told me that I have a tooth problem." (P3) "I did physiotherapy for several months; the doctor told me that you have cervical radiculopathy." (P19).
Subtheme 2.2: Life-Threatening Errors
- In some cases, misinterpretation of symptoms led to invasive and anxiety-provoking investigations:
"I did CT and conventional angiography; they suspected cardiac problems." (P11). - These experiences intensified fear and reinforced perceptions of bodily vulnerability.
- Subtheme 2.3: Chronic Mislabeling: Long-term misdiagnosis resulted in prolonged exposure to ineffective treatments:
- "For the last five years, I have been taking medication for migraine; I did not know I have nTOS." (P22)
- Participants described frustration at years of symptom management without etiological understanding.
Theme 3: Social and Familial Invalidation
Beyond medical encounters, participants reported invalidation within social and familial contexts, often leading to isolation and stigmatization.
Subtheme 3.1: Medical Gaslighting
Several participants perceived dismissal of their symptoms as psychological or fabricated:
"Even I faced social problems; they told me you have no disease, you are a psychopath all those investigations are normal, so you have no disease." (P5).
Such experiences contributed to erosion of trust in healthcare providers.
Subtheme 3.2: Familial Pressure
Family members, influenced by medical uncertainty, frequently encouraged psychiatric consultations:
- "Several times I visited psychiatrist; my family forced me to visit them. I knew that I have a somatic problem." (P17).
- This dynamic amplified patients’ sense of not being believed.
Theme 4: Profound Emotional Suffering
- The cumulative effect of diagnostic delay, mislabeling, and invalidation resulted in marked emotional distress.
- Subtheme 4.1: Isolation and Despair
- Participants expressed persistent feelings of loneliness, hopelessness, and emotional exhaustion:
- "I have been very upset as no one understands my suffering." (P9).
- Themes of hopelessness and emotional despair were identified in 84% of transcripts, underscoring the psychological toll of prolonged diagnostic uncertainty.
To facilitate interpretation, a conceptual model was developed to visually represent the cumulative and distressing diagnostic journey experienced by participants (Figure 1), integrating clinical, emotional, and social dimensions of the illness trajectory.
Discussion
Neurogenic TOS acts as a "diagnostic chameleon," with symptoms mimicking many common conditions. It presents a significant diagnostic challenge because its symptoms overlap extensively with a wide range of neurological, musculoskeletal, and systemic conditions, making differential diagnosis complex. Cervical spine pathology, particularly cervical radiculopathy due to disc herniation, spondylosis, or foraminal stenosis, is one of the most important differentials, as it can produce neck pain radiating to the upper limb, paresthesia, weakness, and sensory disturbances that mimic nTOS. Peripheral nerve entrapment syndromes such as carpal tunnel syndrome (median nerve), cubital tunnel syndrome (ulnar nerve), and radial tunnel syndrome may coexist with or masquerade as nTOS [6,7].
Brachial plexopathies due to trauma, tumors, radiation injury, or inflammatory processes must also be considered, particularly when symptoms are progressive, asymmetric, or associated with objective neurological deficits. In addition, Shoulder pathologies – such as rotator cuff disease, adhesive capsulitis, and impingement syndrome, commonly present with pain exacerbated by arm elevation and are frequently misinterpreted as thoracic outlet pathology. Myofascial pain syndromes, especially involving the scalene, trapezius, or pectoralis minor muscles, can closely mimic nTOS, contributing to pain, heaviness, and paresthesia without clear structural compression. Rheumatological conditions such as fibromyalgia, inflammatory arthritis, or connective tissue diseases may produce widespread pain, fatigue, and sensory symptoms [6,7].
Given this broad differential, the diagnosis of nTOS remains largely clinical, dependent on meticulous history-taking, thorough physical examination, exclusion of alternative diagnoses, and a multidisciplinary approach to avoid misdiagnosis and prolonged patient suffering [8,9].
Misdiagnosis in nTOS is not benign. In the present cohort, incorrect diagnostic attribution resulted in iatrogenic harm, including unnecessary dental extractions and invasive investigations such as conventional angiography. These findings underscore how diagnostic uncertainty can escalate into physical harm, psychological distress, and avoidable healthcare expenditures an issue increasingly recognized in patient safety literature.
Beyond biomedical consequences, participants described profound social invalidation in chronic illness contexts embodies the stigma of "invisible illnesses," where the absence of visible markers or abnormal test results often leads healthcare providers and society to attribute symptoms to psychosomatic causes, thereby undermining patient credibility and autonomy. This medical invalidation involves dismissal or minimization of patient reports, particularly among women, or young adults (typically nTOS patients), fostering a cycle of doubt that erodes patient agency. Medical invalidation often manifests as minimization or dismissal of patient-reported symptoms, particularly when diagnostic tests are normal. Rather than resolving uncertainty, normal results may paradoxically intensify clinician skepticism, shifting explanatory frameworks from physiological to psychological domains. This process can lead to diagnostic overshadowing, patient self-doubt, and erosion of trust in healthcare relationships [10,11]. The emotional suffering described by participants closely parallels Joseph Dumit's concept of "illnesses you have to fight to get treated," drawn from analyses of multiple chemical sensitivity (MCS), where patients face systemic barriers in uncertain, emergent illnesses, with bureaucratic and institutional reliance on established biomarkers denying legitimacy and forcing collective advocacy to counter exclusions. This fight manifests in newsgroup archives and patient activism, revealing rhetorical tactics by institutions that exploit scientific open-endedness to reject emerging facts, perpetuating costly struggles for recognition. Invalidation extends beyond clinics to social services and workplaces, with high discounting reported in insurance interactions, hindering return-to-work efforts and amplifying shame, while gender and minority intersections intensify stigma through diagnostic overshadowing leading to loneliness, helplessness, and mental health crises [10-13].
Clinical implications for managing nTOS emphasize rigorous training on key red flags to facilitate early detection and intervention, including positional symptoms such as pain, paresthesia, or weakness exacerbated by overhead arm positions, overhead activities, or specific postures like carrying heavy loads, alongside provocative tests like the elevated arm stress test (EAST). Other critical red flags encompass supraclavicular tenderness, thenar atrophy signaling chronic median nerve involvement, positive Adson or Wright maneuvers eliciting radial pulse diminution or symptom reproduction, and vascular signs like hand pallor or Raynaud-like phenomena during maneuvers. Multidisciplinary TOS clinics, integrating vascular surgeons, neurologists, physiatrists, physical therapists, and pain specialists, may cut diagnostic and treatment delays significantly potentially by streamlining evaluations via coordinated imaging (MRI neurography, dynamic venography), standardized protocols, and surgical referrals addressing the typical 1-2 year lag from symptom onset to definitive care that perpetuates deconditioning, psychological distress, and suboptimal outcomes in this anatomically complex syndrome involving scalene hypertrophy, cervical ribs, or fibromuscular bands. Evidence supports such clinics reducing misdiagnoses (e.g., labeling nTOS as "psychosomatic" or shoulder pathology), improving surgical success rates to 80-90% via first-rib resection or scalenectomy when conservative measures like physical therapy, nerve blocks, or Botox fail, and enhancing patient-centered metrics including return-to-work timelines and quality-of-life scores through holistic management that tackles myofascial triggers, posture correction, and psychosocial support. Implementing these in high-volume centers could standardize care, lower healthcare costs from repeated consultations [14-18].
Future directions in nTOS research prioritize mixed-methods studies incorporating male patients to address current gender imbalances, as existing literature predominantly features female cohorts due to higher reported prevalence and referral biases, potentially overlooking male-specific biomechanics like broader clavicular spans, occupational overhead strain in trades, or delayed presentations from stoicism, thereby enabling qualitative insights into lived experiences alongside quantitative metrics on symptom trajectories, diagnostic timelines, and treatment responses to refine inclusive diagnostic algorithms and prognostic models. Such studies could employ patient diaries, focus groups, and wearable sensor data to capture nuanced positional triggers absent in traditional cohorts, while longitudinal tracking via standardized scales (e.g., DASH for disability, SF-36 for quality-of-life) elucidates sex-disaggregated outcomes post-scalenectomy or therapy, informing tailored rehabilitation protocols that mitigate male underdiagnosis risks like conflation with rotator cuff pathology. Concurrently, intervention trials targeting clinician education represent a critical frontier, testing structured curricula to enhance recognition of red flags like supraclavicular tenderness or thenar wasting, countering few years diagnostic odyssey driven by misattribution to psychogenic causes. Randomized controlled trials could randomize primary care providers or neurologists to intervention arms versus controls, measuring endpoints like referral accuracy to multidisciplinary TOS clinics, reduction in unnecessary MRIs, and patient-reported delays, with embedded knowledge translation via pre/post assessments and fidelity checks to ensure scalability across community and academic settings [14,16-18].
This study has several limitations. The female-only sample limits generalizability to male populations. Retrospective accounts are subject to recall bias, and recruitment from a single specialty clinic may introduce selection bias. Nonetheless, the depth and consistency of narratives provide valuable insight into the lived experience of diagnostic delay in nTOS.
Conclusion
Delayed or missed diagnosis of nTOS inflicts multilayered suffering, extending beyond physical symptoms to encompass emotional distress, social invalidation, and erosion of trust in healthcare systems. The findings underscore the urgent need for a shift toward syndrome-aware, patient-centered, and empathetic diagnostic approaches that recognize both the clinical complexity of nTOS and the lived experiences of affected individuals. Early validation of patient narratives combined with rigorous clinical assessment and multidisciplinary collaboration has the potential to shorten diagnostic pathways, reduce iatrogenic harm, and transform prolonged diagnostic odysseys into meaningful trajectories of recognition, relief, and recovery.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Written informed consent was obtained from all patients and/or their legal guardians for participation in this study and for the publication of any accompanying images, clinical information, and other data included in the manuscript.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: SHM and BAA were major contributors to the conception of the study, as well as to the literature search for related studies. FHK, YNA and AKG were involved in the literature review and the writing of the manuscript. SKA, NSS, LJM, ASH, CSO, AHA, OMH, LAS, AAM, and AHH were involved in the literature review, the design of the study, the critical revision of the manuscript, and the preparation of the table and figure. FHK and BAA confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Use of AI: ChatGPT Plus (OpenAI, version 4) was used solely to assist with language editing and to improve the clarity of the manuscript. All content was carefully reviewed and verified by the authors, who take full responsibility for the accuracy, integrity, and originality of the entire work.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Divergent Conceptualizations and Management Strategies for Neurogenic Thoracic Outlet Syndrome: A Qualitative Multispecialty Study
Fahmi H. Kakamad, Saywan K. Asaad, Abdullah K. Ghafour, Azad Star Hattam, Lawand Ahmed Sharif,...
Abstract
Background
Neurogenic thoracic outlet syndrome (nTOS) is the most prevalent subtype of thoracic outlet syndrome and remains one of the most controversial conditions in peripheral nerve and thoracic disorders. Despite widespread recognition of conservative therapy as initial management, substantial variation exists across medical specialties regarding diagnosis, duration of nonoperative treatment, and indications for surgery. These discrepancies suggest underlying differences in how nTOS is conceptualized rather than disagreement over available treatment options.
Objectives
This study aimed to explore and compare the perspectives of different medical specialties on the management of confirmed nTOS, with particular attention to conservative therapy, surgical indications, and underlying explanatory models.
Methods
A qualitative descriptive study was conducted using semi-structured interviews with 40 physicians from five specialties involved in nTOS care: thoracic and vascular surgery, neurosurgery, orthopedic surgery, neurology, and rheumatology (eight participants per specialty). Participants were recruited using purposive sampling based on clinical experience with nTOS. All interviews centered on a standardized question addressing management strategies following confirmation of nTOS. Data were analyzed using reflexive thematic analysis.
Results
Five overarching themes emerged. All specialties endorsed physiotherapy as first-line treatment, though recommended duration varied widely. Profound disagreement existed regarding the role of surgery, ranging from early operative intervention to complete rejection. Surgeons tended to frame nTOS as a mechanical compression disorder, whereas neurologists and rheumatologists frequently expressed diagnostic skepticism and favored prolonged conservative management. Orthopedic surgeons adopted selective surgical strategies focused on musculoskeletal contributors. Across specialties, variability was driven primarily by differing conceptual models of nTOS rather than by technical considerations.
Conclusion
Management variability in nTOS arises chiefly from divergent understandings of the condition itself. Without addressing these foundational differences, inconsistency in care is likely to persist. Interdisciplinary consensus-building that integrates anatomical, neurological, and pain-based frameworks is essential for developing coherent, patient-centered management pathways for nTOS.
Introduction
Neurogenic thoracic outlet syndrome (nTOS) is the most common and arguably the most controversial subtype of thoracic outlet syndrome (TOS), accounting for more than 90% of reported cases. It is characterized by compression of the brachial plexus as it traverses the thoracic outlet, leading to a constellation of symptoms including neck and shoulder pain, upper limb paresthesia, weakness, fatigue, and functional impairment. Despite its relatively high prevalence compared with vascular forms of TOS, nTOS remains poorly understood, frequently underdiagnosed, and inconsistently managed across medical specialties [1-3].
Management of nTOS is contentious. Conservative treatment, including physiotherapy, postural correction, pain management, and behavioral modification, is generally recommended as first-line therapy. However, the indications for surgical intervention, optimal timing, patient selection, and preferred surgical approach remain subjects of ongoing debate. Surgical decompression most commonly involving first rib resection and scalenectomy has been reported to yield favorable outcomes in selected patients, yet reported success rates vary widely, and complications are not negligible. These uncertainties contribute to divergent management philosophies across specialties involved in nTOS care [4-7].
The multidisciplinary nature of nTOS care further complicates consensus. Thoracic and vascular surgeons often approach nTOS from an anatomical and decompressive perspective, emphasizing surgical solutions in carefully selected patients. Neurosurgeons may focus on neural pathology, central sensitization, and differential diagnoses involving cervical spine or peripheral nerve disorders. Orthopedic surgeons frequently view symptoms through the lens of musculoskeletal dysfunction, shoulder pathology, or cervical spine disease. Neurologists may prioritize electrodiagnostic findings and are often skeptical of nTOS in the absence of objective abnormalities. Rheumatologists, meanwhile, may encounter patients with overlapping pain syndromes or inflammatory conditions, influencing their perception of nTOS as a diagnosis of exclusion. These differing conceptual frameworks shape not only clinical decision-making but also attitudes toward diagnosis, referral, and treatment [8-11].
While numerous quantitative studies have evaluated surgical outcomes, diagnostic tests, and rehabilitation protocols in nTOS, relatively little attention has been paid to the perspectives of clinicians themselves. Understanding how different specialties conceptualize nTOS, interpret evidence, and justify their management strategies is critical, as these views directly influence patient pathways, interdisciplinary collaboration, and ultimately clinical outcomes. Qualitative research is particularly well suited to exploring such complex, context-dependent phenomena, allowing for in-depth examination of beliefs, experiences, uncertainties, and professional cultures that cannot be adequately captured through quantitative methods alone [12].
Therefore, this qualitative study aims to explore and compare the views of different specialties (thoracic and vascular surgeons, neurosurgeons, orthopedic surgeons, neurologists, and rheumatologists) regarding the management of nTOS. By elucidating areas of consensus, disagreement, and uncertainty across specialties, this research seeks to inform more coherent multidisciplinary approaches, identify barriers to collaboration, and contribute to the development of more patient-centered and evidence-informed care pathways for individuals with nTOS.
Methods
Study design
This study adopted a qualitative descriptive design to explore how different medical specialties manage confirmed cases of nTOS. Given the complexity, controversy, and specialty-dependent interpretations surrounding nTOS, a qualitative approach was chosen to capture clinicians’ reasoning, preferences, and professional perspectives that cannot be adequately quantified.
Participants and sampling
Physicians from five specialties commonly involved in the care of nTOS were included: thoracic and vascular surgery, neurosurgery, orthopedic surgery, neurology, and rheumatology. Participants were recruited using purposive sampling based on their direct clinical experience with patients diagnosed with nTOS.
A total of 40 clinicians participated in the study. Each specialty was represented by eight participants, ensuring sufficient diversity of viewpoints within and across specialties. Participants varied in years of experience and practice settings, enhancing the richness of the data.
Data collection
Data were collected through semi-structured interviews. All participants were asked a single core, standardized question to ensure comparability across specialties:
“If you confirm that a patient has neurogenic thoracic outlet syndrome, how do you manage this patient?”
Follow-up prompts were used when needed to clarify responses, particularly regarding duration of conservative therapy, indications for surgery, and preferred surgical approaches. Interviews were conducted in person or online, recorded with consent, and transcribed verbatim for analysis.
Data analysis
Data were analyzed using reflexive thematic analysis as described by Braun and Clarke [12]. Analysis proceeded through the following phases:
- Familiarization with the data through repeated reading of transcripts
- Generation of initial codes reflecting management strategies, attitudes toward surgery, and specialty-specific reasoning
- Development of preliminary themes across and within specialties
- Review and refinement of themes to ensure internal coherence and clear distinction
- Definition and naming of final themes
Analysis was conducted iteratively, with reflexive attention to how professional background and clinical culture shaped interpretations. Discrepancies and contradictions were treated as meaningful data rather than inconsistencies.
Ethical considerations
Participation was voluntary, and informed consent was obtained from all participants. All data were anonymized to protect participant identity. The study involved clinicians only and did not include patient data.
Results
Analysis revealed marked variation in the management of nTOS across specialties, particularly regarding the role of surgery, duration of conservative treatment, and confidence in the diagnosis itself. Five overarching themes emerged.
Theme 1: Universal Endorsement of Physiotherapy as First-Line Treatment
Across all specialties, physiotherapy was consistently identified as the initial management strategy for confirmed nTOS. However, the recommended duration varied substantially, ranging from two weeks to six months.
- Thoracic and vascular surgeons most commonly recommended 1–3 months of physiotherapy.
- Orthopedic surgeons and neurologists often advocated prolonged physiotherapy (up to six months).
- Rheumatologists generally supported physiotherapy as the primary or sole treatment.
This variation reflects differing thresholds for declaring conservative treatment failure.
Theme 2: Profound Disagreement Regarding the Role of Surgery
Opinions on surgical intervention ranged from early and decisive to complete rejection.
Thoracic and Vascular Surgeons:
This group demonstrated the widest internal variability:
- Some favored early surgery, even direct operative intervention after short physiotherapy trials.
- Others recommended surgery only after structured conservative management.
- A minority rejected surgery entirely, citing poor outcomes and limited benefits.
Preferred surgical approaches included supraclavicular, transaxillary, and minimally invasive video assisted thoracic surgery/robotic assisted thoracic surgery-VATS/RATS) first rib resection, often tailored to venous involvement or pectoralis minor tenderness.
Neurosurgeons:
Neurosurgeons were divided:
- Some viewed nTOS as a clear anatomical compression requiring surgery.
- Others avoided intervention entirely, preferring referral to vascular surgeons.
- Posterior approaches were rarely mentioned and limited to individual preferences.
Theme 3: Selective and Limited Surgical Indications Among Orthopedic Surgeons
Orthopedic surgeons generally adopted a conservative and selective surgical philosophy:
- Surgery was reserved for chronic cases, documented anatomical abnormalities, or failure of extended physiotherapy.
- Several rejected first rib resection, favoring isolated scalenectomy or pectoralis minor tenotomy.
- A minority supported direct surgical referral to vascular surgeons.
This reflects the musculoskeletal framing of nTOS symptoms.
Theme 4: Skepticism and Diagnostic Reframing by Neurologists
Neurologists frequently expressed diagnostic skepticism even when they are asked regarding the management of nTOS:
- Several participants attributed symptoms to migraine, central sensitization, or non-structural causes.
- Surgery was considered only in cases with objective findings, such as muscle atrophy.
- Most recommended prolonged conservative therapy, with surgical referral as a last resort.
This group demonstrated the highest threshold for surgical acceptance.
Theme 5: nTOS as a Diagnosis of Exclusion Among Rheumatologists
Rheumatologists often conceptualized nTOS as a diagnosis of exclusion:
- Most endorsed physiotherapy initially.
- Surgical referrals were deferred to vascular surgeons and often viewed with skepticism.
- Some explicitly stated that surgery is ineffective or unnecessary.
This perspective reflects overlap with chronic pain syndromes and inflammatory conditions (Table 1).
|
Specialty |
Conceptual Model of nTOS |
Conservative Management |
View on Surgery |
Typical Surgical Preference |
|
Thoracic & Vascular Surgeons |
Mechanical compression |
Short–moderate physiotherapy |
Broadly supportive, variable timing |
First rib resection (SC, TA, VATS/RATS) |
|
Neurosurgeons |
Neural compression vs central causes |
Variable duration |
Divided; selective or avoided |
Rare; referral preferred |
|
Orthopedic Surgeons |
Musculoskeletal dysfunction |
Prolonged physiotherapy |
Highly selective |
Scalenectomy or PM tenotomy |
|
Neurologists |
Diagnostic skepticism |
Prolonged conservative care |
Rare; last resort |
Only with objective deficits |
|
Rheumatologists |
Diagnosis of exclusion |
Primary or sole treatment |
Generally opposed |
Referral to surgeons |
|
SC = supraclavicular; TA = transaxillary; VATS = video-assisted thoracic surgery; RATS = robotic-assisted thoracic surgery; PM = pectoralis minor. |
||||
Discussion
The study illustrates that variability in the management of nTOS is driven less by disagreement over treatment modalities and more by fundamentally different ways in which clinicians understand the condition itself rather than reflecting simple differences in training, the observed diversity in management strategies appears rooted in contrasting explanatory models of nTOS, ranging from structural compression to functional or centrally mediated pain syndromes. Similar conceptual fragmentation has been repeatedly identified as a central challenge in advancing care for nTOS [13–15].
Although conservative management was universally endorsed, the absence of shared criteria for adequacy or failure of nonoperative therapy emerged as a critical fault line between specialties. The wide range of physiotherapy durations recommended by participants suggests conservative treatment functions as both therapy and diagnostic test, with clinicians using response to rehabilitation to validate or refute the diagnosis. This implicit diagnostic role of physiotherapy has been noted in previous studies and may partly explain why treatment pathways diverge early in the disease course [16,17]. Importantly, prolonged conservative management may delay surgical referral in patients who could potentially benefit from decompression, while premature escalation risks unnecessary intervention.
Disagreement surrounding surgery reflects ongoing uncertainty regarding the pathophysiology of nTOS rather than technical differences in operative approach. Surgeons who favored intervention generally conceptualized nTOS as a mechanical compression disorder, whereas those opposing surgery questioned the causal relationship between anatomical findings and symptoms. This division mirrors inconsistencies in the literature, where anatomical abnormalities are common in asymptomatic individuals and clinical improvement does not always correlate with radiological or intraoperative findings [1,11,18,19]. Consequently, surgical decision-making remains heavily dependent on clinician judgment rather than objective thresholds.
Specialty-specific skepticism, particularly among neurologists and rheumatologists, highlights the tension between symptom-based diagnoses and disciplines that prioritize objective biomarkers. Neurogenic TOS challenges traditional diagnostic paradigms because standard electrodiagnostic studies are often normal and imaging findings are nonspecific [20,21]. From a neurological or rheumatological standpoint, this diagnostic ambiguity fosters reinterpretation of symptoms as functional, inflammatory, or centrally mediated disorders. While such caution is justifiable, it may inadvertently marginalize patients whose symptoms do arise from peripheral neural compression.
The selective surgical stance adopted by many orthopedic surgeons reflects a musculoskeletal framing of nTOS, emphasizing regional biomechanics over thoracic outlet anatomy. This perspective aligns with growing interest in pectoralis minor syndrome and isolated scalene pathology as contributors to upper limb symptoms [22,23]. However, the lack of consensus on whether these entities represent distinct conditions or part of the nTOS spectrum further complicates interdisciplinary communication and treatment planning.
A notable implication of these findings is that nTOS lacks a shared “clinical ownership.” Instead, responsibility is frequently transferred between specialties, resulting in circular referrals and inconsistent care pathways. Similar patterns have been described in other contested pain syndromes and are known to contribute to patient dissatisfaction and healthcare inefficiency [24,25]. Multidisciplinary evaluation has been proposed as a solution, but without alignment at the conceptual level, such models risk becoming parallel rather than integrative.
There are limitations to this study; The study reflects clinicians stated practices rather than observed behavior, and responses may have been influenced by recall or professional positioning. Additionally, perspectives may vary across healthcare systems. Nevertheless, the consistency of themes across specialties suggests that the findings capture widely held views rather than isolated opinions.
Implications for Practice and Research
Future efforts should focus on developing interdisciplinary definitions of conservative treatment failure, clearer indications for surgical referral, and shared diagnostic language. Consensus statements that integrate anatomical, neurological, and pain-based frameworks may help bridge existing divides. Further qualitative work involving patients may also clarify how professional disagreement translates into lived experience.
Conclusion
This study demonstrates that disagreement in nTOS management arises primarily from divergent conceptualizations of the condition rather than lack of therapeutic options. Without addressing these underlying differences, variability in care is likely to persist. Meaningful progress in nTOS management will depend on sustained interdisciplinary engagement aimed at reconciling competing models into coherent, patient-centered care pathways.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: SHM and BAA were major contributors to the conception of the study, as well as to the literature search for related studies. FHK, SKA and AKG were involved in the literature review and the writing of the manuscript. ASH, LAS, HSN, RHA, ZOKA, SSR, SOA, NSS, CSO, LJM, and YKI were involved in the literature review, the design of the study, the critical revision of the manuscript, and the processing of the table. FHK and BAA confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Use of AI: ChatGPT Plus (OpenAI, version 4) was used solely to assist with language editing and to improve the clarity of the manuscript. All content was carefully reviewed and verified by the authors, who take full responsibility for the accuracy, integrity, and originality of the entire work.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Review Articles
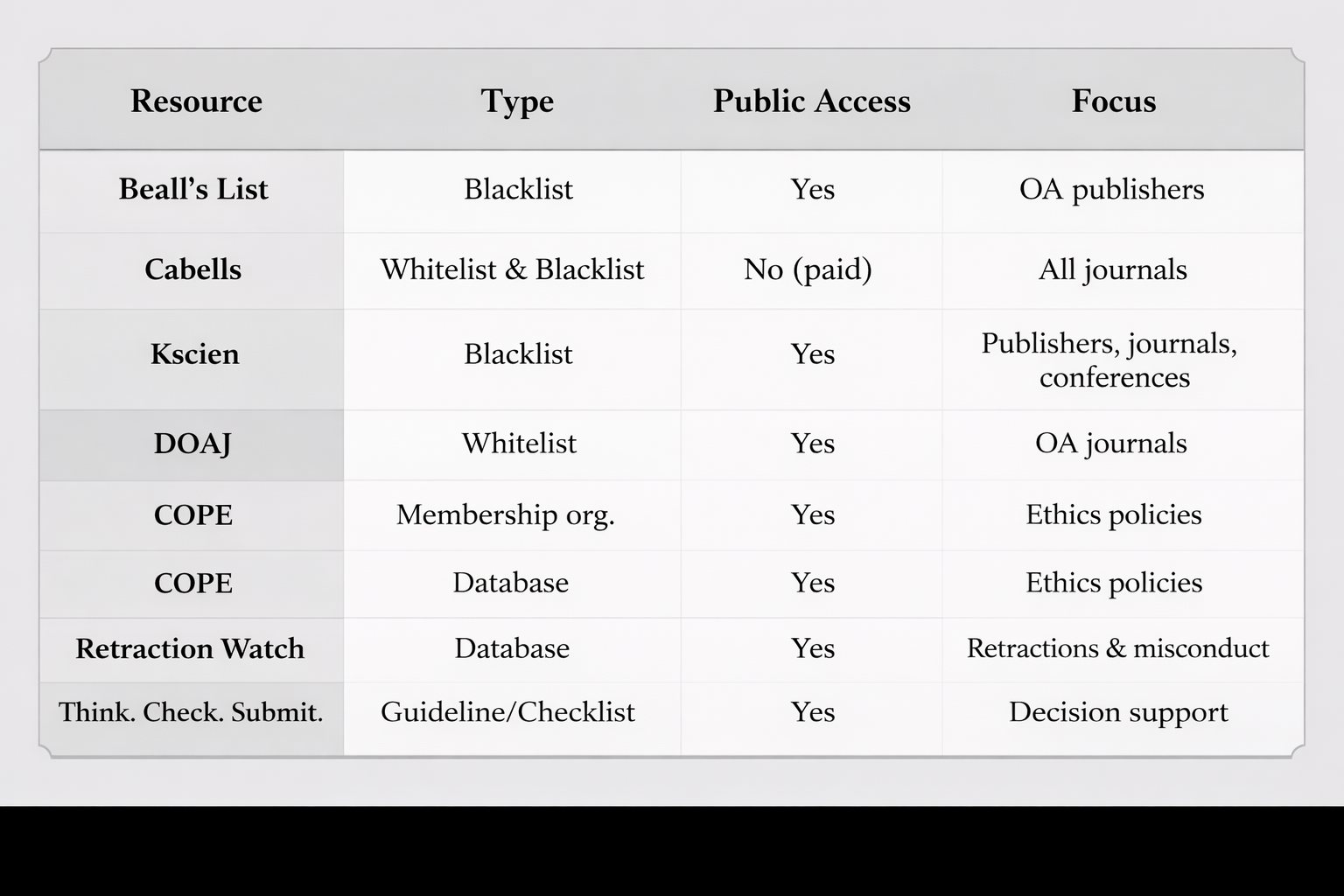
Renaming the Problem: Why ‘Non-Recommended Journals’ Is Preferable To ‘Predatory’ in Academic Publishing
Frederick M. Tiesenga, Daniel Rodger, Benjamin Saracco, Baichang Zhong, Andrea Cortegiani,...
Abstract
The term "predatory journals" is widely used to describe publishing practices that exploit authors, compromise research quality, and mislead readers. Its use, however, has frequently led to legal threats and professional conflicts for individuals and institutions who call out such deceptive practices. Most notably, Jeffrey Beall, the creator of Beall’s List, faced legal threats and personal harassment, which ultimately led him to discontinue his work. To address these challenges, scholars have proposed replacing “predatory journals” with more neutral alternatives, such as “questionable journals”. This study recommends using the term “non-recommended journals,” which similarly avoids accusatory language while signaling the need for caution by scholars and institutions. By avoiding direct allegations of unethical conduct, the term "non-recommended" reduces the likelihood of legal repercussions and professional disputes. Adopting this terminology enables researchers and institutions to continue addressing concerns about low-quality or deceptive publishing practices while fostering a more constructive dialogue. This reframing encourages constructive dialogue, broader institutional engagement, and stronger collective efforts to uphold high ethical publishing standards and protect academic integrity.
Introduction
Since the 1990s, scholarly publishing has undergone a significant transformation from a subscription-based print model to a digital, open-access framework [1]. However, this shift has been accompanied by the rise of unethical and deceptive publishing practices [2]. Predatory journals, typically operating on a ‘pay-to-publish’ model, exploit the open-access system primarily for financial gain, prioritizing profit for their editor–owners rather than maintaining scholarly integrity [3].
In 2019, a panel of scholars and publishers from ten countries established a consensus definition of predatory publishing aimed at protecting the integrity of scholarly communication. According to this definition, predatory journals and publishers are “Entities that prioritize self-interest at the expense of scholarship and are characterized by false or misleading information, deviation from best editorial and publication practices, a lack of transparency, and/or the use of aggressive and indiscriminate solicitation practices” [4]. Such journals often employ unethical practices, including persistent and unsolicited requests for submissions, inadequate or entirely absent peer review despite claims to conduct it, opaque or excessive publication charges, and poor editorial or technical standards. Most importantly, their failure to ensure rigorous peer review threatens the credibility and trustworthiness of the scientific record [4].
The impact of these journals extends across a broad spectrum of researchers, affecting not only readers and early-career, inexperienced, or uninformed scholars, particularly those from developing nations and high- to upper middle-income countries, but also well-established academicians [5,6]. In response, various blacklists, whitelists, and institutional guidelines have been developed to help researchers identify these journals.
The continued use of the term "predatory journals" has created conflicts and legal challenges for individuals and organizations addressing these practices. Jeffrey Beall, a librarian at the University of Colorado, curated "Beall's List," a compilation of potential predatory open-access publishers [3]. In 2013, the OMICS Publishing Group, featured on this list, threatened Beall with a 1 billion $ defamation lawsuit, leading him to feel "personally threatened" [7]. Similarly, the Canadian Center of Science and Education accused him of defamation, labeling his list as "actionable libel" and challenging his recommendations against engaging with certain publishers [3].
Importantly, Beall faced online harassment, including websites that attacked his character, labeling him an "academic terrorist" and making unfounded personal accusations [8]. Institutions such as the University of Montreal and initiatives like Cabells Predatory Reports have also faced lawsuits and threats. To mitigate these conflicts, we support the proposal of Kakamad et al. in the 18th general assembly of the European Association of Science Editors (EASE) to replace the term "predatory journals" with "non-recommended journals" [9].
In this article, we examine the historical and linguistic evolution of the term “predatory”, evaluating its institutional and ethical implications, and propose “non-recommended” journals as a pragmatic and defensible alternative. By analyzing the trajectory of terminology and policy responses, we argue that adopting more neutral language can help to protect academic integrity while reducing legal and reputational risks.
The Evolution of "Predatory": From Plunder to Modern Exploitation
The term "predatory" is deeply associated with exploitation and harm, evolving from its original meaning of physical plundering to its modern usage across various domains, including finance, publishing, and interpersonal interactions. The word "predatory" originates from the Latin "praedator," meaning "plunderer," which comes from "praedare" ("to plunder") and "praeda" ("prey"). Its earliest documented use in English dates to the late 1580s, describing acts of plundering or pillaging. This establishes its historical link to aggressive acquisition and territorial violation. By the 1660s, the term extended to zoology, describing animals that "habitually prey upon other animals." This shift expanded its meaning beyond human acts of looting to natural behaviors in the animal kingdom [10, 11].
The related term "predation" first appeared in the late 15th century as "predacioun," meaning "act of plundering," from the Latin "praedationem" ("a plundering") and "praedari" ("to rob"). The word "predator," specifically referring to an animal that preys on others, entered English in 1862. This relatively late adoption suggests a growing interest in the scientific study of animal behavior. Wiktionary traces "predator" back to the Latin "praedātor," meaning "loot" or "pillage." The word "prey" has an equally long history, dating to the mid-13th century as "preie," meaning "animal hunted for food." It was also used metaphorically to describe "souls captured by Satan" or "goods taken in war," stemming from the Old French "preie" and Latin "praeda," meaning "booty" or "game hunted." This linguistic evolution reinforces the concept of exploitation inherent in the term "predatory" [11, 12]
In modern times, "predatory" has expanded to describe unethical or exploitative practices in multiple fields. "Predatory lending" refers to abusive loan terms imposed on vulnerable borrowers, while "predatory pricing" describes pricing strategies intended to eliminate competition. Vocabulary.com defines a predator as "an animal that eats other animals, or people or companies who act like they do," illustrating its figurative application [13, 14]. One of the most significant contemporary uses of "predatory" is in academic publishing. Initially coined by Beall in 2010 [15,16].
Tracing the Origins of Predatory Publishing
The issue of what is now recognized as predatory publishing was first addressed as early as 2008. For instance, Gunther Eysenbach wrote a blog post [17], and Katharine Sanderson published an article [18], both discussing the prevalence of low-quality and potentially fraudulent publishing practices. They described such publishers using terms like "black sheep among open-access publishers" [17,18].
Beall’s early works on predatory publishing, all published in the Charleston Advisor, examined and analyzed several publishers. Of the 18 publishers discussed, only one was not categorized as predatory. In his first paper [19], Beall focused on Bentham Open, detailing its practices, such as charging membership and article processing fees, indexing methods, and search functionality on its website. He pointed out that Bentham Open published 236 journals, most of which featured articles that Beall deemed of low quality, suggesting they would likely not have been accepted by higher-tier journals. Because the journals were less than three years old, none yet had an impact factor. Beall concluded that Bentham Open, which entered scholarly publishing in 2007, primarily served as a platform for disseminating research of dubious quality. He argued that the publisher exploited the open access model for financial gain and inundated the scholarly community with substandard and questionable research [19].
In April 2010, Beall published another article, marking the first instance where he introduced the term "predatory" in a scholarly context. This article examined an additional nine publishers, with publication fees varying between $99.95 and $1,699. However, due to the differing pricing structures, direct comparison across all publishers was challenging and four of the nine publishers did not disclose their fees. Each publisher was assessed based on four criteria: Content, User Interface/Searchability, Pricing, and Contract Options. In this article, Beall highlighted that he was not the only one recognizing this emerging trend in academic publishing. He referred to prominent figures in the open access movement, including Stevan Harnad, who had also begun to criticize its implications. Beall cited Harnad’s blog, which discussed the increasing prevalence of rapidly established gold open-access journal networks. These journals often lack substantial scholarly or publishing expertise and primarily rely on aggressive online solicitation [20, 21]. In 2010, Beall published another paper analyzing three additional predatory publishers [22]. Then, in 2012, he expanded his investigation to include five more publishers. Of these, Beall identified four as predatory, while one was deemed legitimate [23]. These four studies collectively examined 18 publishers, responsible for publishing 1,328 journals at the time.
In 2013, Beall published his final article on this topic [24], focusing not only on publishers, but on specific journals, such as British Journal of Science, International Journal of Current Research, International Journal of Science and Advanced Technology, International Journal of Sciences, and World Journal of Science and Technology. Beall highlighted that these journals operated independently without a publisher, had broad scopes, minimal peer review, and seemed to prioritize quantity over quality by accepting as many papers as possible. His analysis pointed out several red flags now associated with potentially fraudulent behavior, such as misleading contact information, false or unclear details about the country of origin, websites with sparse content, editorial boards that appeared fabricated, misleading or absent impact factors, poor language, and the assignment of copyrights to journals even though authors paid to publish [24]. Between 2009 and 2018, Beall authored 40 articles addressing the issue of predatory publishing. Many of these were brief reviews, likely invited, that highlighted the risks associated with such practices. Some articles focused on the specific issues within the open-access model, while others were published in discipline-specific journals [15].
The Predatory Lists
Beall’s list
In 2010, Beall created his first blog, which listed fewer than 20 publishers, but this initial list was largely ignored [15]. By 2012, Beall transitioned the blog to a WordPress platform, renaming it “Scholarly Open Access” [25] though it is more commonly referred to as “Beall’s List.” The blog included a “Watchlist,” and was, although being listed there, often viewed as equivalent to the main list [25]. He decided to create his list after receiving numerous unsolicited invitations to join editorial boards of various journals. Initially, the list garnered little attention but gained significant recognition among academicians by the mid-2010s. The entries on Beall’s list were organized into categories or sub-lists: suspicious publishers, predatory stand-alone journals, and journals that had hijacked legitimate ones [26]. An archived version of the list is available at Beallslist.net, which continues to be updated with notes on the original entries and new additions [27].
Kscien’s list
Following the discontinuation of Beall's list, the non-profit organization Kscien, based in Iraq, took on the responsibility of developing its list of predatory publishers. The creation and ongoing maintenance of this list fall under the purview of the "Predatory List Committee," which is composed of several emerging researchers dedicated to tracking and identifying the evolving strategies and tactics employed by predatory entities. The criteria for identifying such practices focus on journal misconduct, fabrication, and the presence of inadequate peer review procedures [28]. Initially, Kscien's list mirrored Beall’s list by categorizing entities into four distinct groups: "predatory publishers," "predatory stand-alone journals," "hijacked journals," and "misleading metrics" [28]. As predatory practices continue to evolve, Kscien has expanded the scope of its list by introducing two additional categories: the "Conference List" and the "Cumulative List." The "Conference List" highlights predatory conferences, whether independently organized or associated with specific institutions. The "Cumulative List" designed to compile and track all journals associated with predatory publishers in one place, providing a centralized resource for researchers to identify potentially risky publication venues [29]. The list is available at: https://kscien.org/predatory-publishing/
Cabells’ lists
Following the cessation of Jeffrey Beall's lists in 2017, another list maintained by Cabell Publishing Co. (Cabells), a U.S.-based organization, emerged as an alternative, aiming to provide a reliable resource in the same domain. Cabells’ lists, which are primarily based on Beall’s original compilation, were developed. The criteria for Cabells’ lists are comprehensive, encompassing a total of 74 distinct factors, as specified in version 1.1, released on March 13, 2019 [30]. While Beall’s list relied heavily on subjective judgments and limited transparency, Cabells established two curated lists: one identifying journal that meet recognized publishing standards and another identifying those that exhibit deceptive practices. These were originally labeled the “whitelist” and “blacklist,” terms that drew increasing criticism for their racially charged connotations. In response to broader social awareness around racial justice following the murder of George Floyd in 2020, Cabells rebranded the lists as Journalytics (formerly the whitelist) and Predatory Reports (formerly the blacklist) [30, 31].
Predatory Reports
Predatory Reports is an anonymous organization with limited publicly available information, including an unknown establishment date. It has published two lists: the Predatory Journal List and the Predatory Publisher List. According to the organization, it is composed of volunteer researchers who have been affected by the negative impact of predatory publishers. Their mission is to assist researchers in identifying reliable journals and publishers. The organization offers its resources for free to the public, ensuring the information is widely accessible and usable. Operating a website free of advertisements, Predatory Reports is self-funded without external corporate backing. The decision to remain anonymous is due to concerns over potential legal actions from companies with aggressive practices. The organization clarifies that its aim is not to make authoritative claims but to compile and share publicly available information. All published content on its website is backed by referenced reports, allowing individuals to independently assess the material [32].
Several resources have been developed to guide researchers in navigating the complex landscape of scholarly publishing, ranging from blacklists of potentially deceptive journals to whitelists and ethical guidelines. Table 1 provides a summary of these key resources, their types, public accessibility, and focus areas.
|
Resource |
Type |
Public Access |
Focus |
|
Beall’s List |
Blacklist |
Yes |
OA publishers |
|
Cabells |
Whitelist & Blacklist |
No (paid) |
All journals |
|
Kscien |
Blacklist |
Yes |
Publishers, journals, conferences |
|
DOAJ |
Whitelist |
Yes |
OA journals |
|
COPE |
Membership org. |
Yes |
Ethics policies |
|
Retraction Watch |
Database |
Yes |
Retractions & misconduct |
|
Think. Check. Submit. |
Guideline/Checklist |
Yes |
Decision support |
|
OA: Open access, DOAJ: Directory of Open Access Journals, COPE: Committee on Publication Ethics |
|||
Predatory Lists Criticism
Criticism of predatory journal lists often centers on issues of transparency, bias, and the unintended consequences for legitimate publishers. While these lists are designed to protect researchers from deceptive journals, they have faced scrutiny for their methodologies and the broader impact on academic publishing. A notable case is Beall’s list, which was abruptly taken down on January 15, 2017, without warning. Two days later, Andrew Silver reported on its disappearance, prompting speculation about the reasons behind its removal [33]. In a paper published on June 15, 2017, Beall explained that he had deleted his blog due to mounting pressure from his employer and concerns over potential job loss. During the five years that Beall maintained his list, many universities relied on it to advise their researchers against submitting to questionable journals. However, this led to backlash from publishers who sought removal from the list through various means. Some directly contacted Beall, defending their journals, while others escalated their complaints to university officials, including the Chancellor, questioning his ethics and judgment. Beall also faced criticism from the academic librarian community [25]. Other sources have also explored the circumstances surrounding the list’s closure. Basken suggests that Beall faced significant peer pressure, resistance from a Swiss publisher he had listed, and exhaustion from his university, which was frequently targeted with complaints regarding the list [34].
Another issue often raised is that Beall himself made the sole decision regarding which publishers or journals to include on his list. This subjectivity became particularly controversial in 2015 when Frontiers was added to Beall’s list, sparking a debate on social media. One Associate Editor of Frontiers remarked [35], “Frontiers being added to Beall’s list reveals the big weakness of Beall’s list: It’s not based on solid data but on Beall’s intuition.” The editor further argued, “Having a single influential individual cast doubt on such a huge journal feels very unfair”. The same Associate Editor from Frontiers expressed concern that articles published in Frontiers journals might be undervalued due to the publisher's inclusion on Beall's list, suggesting that such articles could be perceived as less valuable because of this association [35]. Some have suggested that this controversy played a significant role in the eventual closure of the list [36]. Teixeira da Silva raised ethical concerns regarding Beall's actions, specifically his decision to remain silent about the reasons for discontinuing his list. Additionally, Beall's failure to issue an apology to those affected, coupled with the resulting void for those who relied on the list for guidance and decision-making, was also criticized. Furthermore, Teixeira da Silva noted that Beall continued to discuss his blog even after its removal [37]. Such ethical debates emphasize the need for transparency and accountability in the management of predatory journal lists to maintain trust within the scholarly community.
Bisaccio highlights that Cabell’s scoring system for its blacklist was specifically designed to prevent the misclassification of legitimate journals, particularly those that are new, from developing countries, or of lower quality, as "predatory "[38]. Another major issue was the criteria and transparency of its evaluations, with some critics arguing that the evaluation process was subjective and lacked transparency. For example, the indicator "no policies for digital preservation" has been criticized for its varying interpretations, and some journals have been blacklisted based on a limited set of criteria, raising concerns about the thoroughness of the evaluation process. Additionally, the list includes numerous "empty journals," or journals that have never published an article, which has led to questions about whether this accurately reflects the status of a journal. There are also concerns about the accuracy and timeliness of the information provided; some journals may be blacklisted without sufficient evidence, while others that engage in questionable practices may be overlooked. The lack of regular updates can lead to outdated information, affecting researchers' decisions [39].
Given these challenges, some scholars argue that focusing on “whitelists” curated collections of journals meeting recognized quality standards, may be a more effective approach than maintaining blacklists. Initiatives such as the Directory of Open Access Journals (DOAJ) and other vetted journal indexes aim to guide researchers toward trustworthy publication venues, thereby achieving the goal of safeguarding scholarly integrity while avoiding the controversies associated with blacklists [38-40].
Concerns About the “Predatory Term"
The term “predatory” has increasingly been challenged by scholars who argue that it may inappropriately label certain journals, particularly those that are low in quality but still legitimate or those that have yet to achieve indexation. Over the past decade, since Beall introduced the concept and term ‘predatory publisher’, his work has elicited both acclaim and criticism in nearly equal measure. While there is broad agreement on the need to address the growing proliferation of low-quality scholarly publications, Beall’s contributions are widely acknowledged as instrumental in initiating efforts to regulate publishing practices and uphold quality and ethical standards in Open Access journals. However, his work has also faced considerable criticism, particularly regarding the terminology and definition of a predatory publisher.
Critics have challenged the use of the term ‘predatory’, arguing that it carries negative connotations and poses a potential threat to academic freedom. As noted by Kimotho, many opponents of Beall’s List view the term as pejorative and problematic. Some dictionaries, including the Cambridge Dictionary, have classified predatory with a “disapproving” usage label. These critics further contend that advocating for a ban on predatory journals may conflict with freedom of speech and restrict researchers' autonomy in selecting publication venues [20, 6, 41]. These perspectives highlight the broader debate surrounding the limitations and ethical implications of labeling journals as "predatory," emphasizing the need for more transparent and objective criteria in evaluating academic publishing practices [15, 37].
Publishers themselves have consistently opposed the use of the term ‘predatory’, and there have been instances where librarians and researchers faced legal action for labeling publishers as potential predators. New [42] recounts a case in which a librarian and his Canadian university employer were sued after the librarian referred to a publisher as “dubious” on his personal blog. Similarly, Todd [43] reported that another Canadian researcher was suspended for identifying certain publishing practices at his institution as predatory, only to be reinstated after a prolonged legal dispute. Even Beall ultimately ceased maintaining his list in 2017 following legal threats [44].
An analysis conducted by Buitrago Ciro and Bowker of 20 university library websites in Canada and the United States revealed that while nearly half of these institutions continued to use the term predatory, many adopted alternative terminology, including deceptive, suspicious, and undesirable. Similarly, Memon notes that terms such as dodgy, fraudulent, pseudo, questionable, sham, and illegitimate have previously been used to describe so-called predatory journals. Beall himself initially employed terms such as perfidious and unscrupulous and continued to incorporate alternative descriptors, including questionable and counterfeit, in later works. Notably, Beall has since acknowledged that the term predatory publisher may not be the most appropriate choice. Reflecting on the persistence of the term nearly seven years later, Beall conceded that it may not be the most precise descriptor of the phenomenon [20, 21, 39,45, 46].
Alternative Terminology
The evolution of concepts often necessitates adjustments in terminology, leading to either refinement or expansion of the original term’s scope. Similarly, no universally accepted definition of "predatory publishing" exists, as scholars debate its criteria. Grudniewicz et al. attempted to define it during the 2019 Predatory Summit in Ottawa, characterizing such entities as those prioritizing self-interest over scholarship through misleading practices and lack of transparency. However, critiques persist regarding the omission of peer review concerns, while Cobey et al. highlight the risks posed by this definitional ambiguity to funding bodies and institutions [4, 47].
Anderson advocated replacing “predatory” with “bad faith” to account for unethical behavior by publishers, authors, and reviewers alike. Similarly, Eriksson et al. propose distinguishing between deceptive and low-quality journals, though Memon argues this distinction remains insufficiently clear. Alternative perspectives suggest that predatory publishers function as parodies, exposing systemic flaws in academic publishing, such as its commercialized nature and biases favoring central institutions over peripheral ones. Furthermore, the concept of predatory practices has expanded beyond publishing to include predatory conferences, authors, and broader concerns about fraudulent science. Although Eriksson et al. introduced a set of comprehensive criteria aimed at eliminating the misnomer “predatory.” They classified journals into two categories: 1) low-quality journals, and 2) deceptive journals. However, these criteria lack the necessary rigor to distinctly separate these two types of journals [20, 6, 39]. Such challenges further demonstrate the need for careful reconsideration of the terms and categories used in scholarly publishing discourse.
Despite its entrenched use, discussions have arisen about whether the term “predatory” should be replaced. At the 2019 Predatory Publishing Summit in Ottawa, Cukier et al. (2020) reported that participants debated alternative terminology. The participants were divided on the issue, with 29% opposing the change, 37% in favor, and 34% remaining neutral. Among the proposed alternatives, dark publisher, deceptive publisher, illegitimate publisher, and publisher operating in bad faith, deceptive publisher received the most support (67%). However, participants recognized significant obstacles to changing the term, including difficulties in literature indexing, dissemination, and the need to update educational materials and funding policies. The summit ultimately concluded that replacing an established term would likely create confusion within the scientific community and hinder progress in addressing the issue [48]. These discussions, along with prior and subsequent milestones in the evolving terminology debate, are summarized in Table 2.
At the 2025 EASE conference, Kakamad et al. introduced and strongly supported the term “non-recommended journals” as a more appropriate alternative to the widely used but controversial term “predatory journals”. The authors argued that while “predatory” suggests malicious intent and deliberate academic misconduct, the term often provokes legal challenges and emotional backlash. They emphasized that “non-recommended” is a neutral, cautious descriptor that avoids passing moral judgment while still guiding researchers away from journals that exhibit low editorial standards, questionable practices, or a lack of rigorous peer review. By doing so, the term encourages wider institutional and individual participation in curbing the spread of poor-quality publishing—without the threat of litigation or reputational damage [9]. This proposal represents the latest milestone in the chronology of terminology development, as illustrated in Table 2. Furthermore, systemic factors can complicate efforts to label journals strictly as “predatory.” For instance, some journals gain inclusion in respected databases such as PubMed not because of rigorous peer review, but due to public research funding, which can inadvertently legitimize research of uncertain quality [49]. In addition, the quality of articles varies widely: some journals traditionally labeled as “predatory” may publish high-quality work when authors submit diligently, whereas even reputable journals occasionally fail to detect flawed or falsified studies [50]. These nuances highlight the challenges of categorical labeling and reinforce the value of adopting a more neutral designation, such as “non-recommended journals,” which encourages careful evaluation of publishing venues without overgeneralization.
|
S. No |
Year |
Event |
Description |
|
1 |
2010 |
Creation of Beall’s List |
Jeffrey Beall, a librarian at the University of Colorado, publishes an online list of suspected predatory journals and publishers. |
|
2 |
2013 |
Widespread Media Attention |
Major outlets such as Nature and The New York Times cover predatory publishing, amplifying global awareness. |
|
3 |
2016 |
OMICS Lawsuit by the U.S. Federal Trade Commission (FTC) |
FTC files a complaint against OMICS Group for deceptive practices, including fake peer review and hidden fees. |
|
4 |
2017 |
Beall’s List Taken Offline |
Beall abruptly takes down his list, allegedly under institutional pressure; the list is later mirrored unofficially. |
|
5 |
2017 |
Cabell’s International Introduces Journal Blacklist |
Cabell’s launches a commercial and curated “Blacklist” of predatory journals, using defined criteria. |
|
6 |
2019 |
UNESCO and Global South Advocates Raise Terminology Concerns |
Scholars call for decolonizing the term "predatory" and exploring more constructive alternatives. |
|
7 |
2020 |
Proposal for Alternative Terminology: “Deceptive Publishing” |
Some experts suggest using less stigmatizing language like "deceptive" or “questionable” journals. |
|
8 |
2022 |
Renewed Academic Debates on Grey Zones in Publishing Practices |
Editorials in Nature, Science, and BMJ Open question binary classifications of journals. |
|
9 |
2025 |
EASE (European Association of Science Editors) Proposal to Mitigate Conflicts and Advance Ethical Publishing |
Shifting from 'Predatory Journals and Publishers' to 'Non Recommended Journals and Publishers |
Limitations and Counterarguments
One clear advantage of the term “predatory” is its moral precision and clarity because it explicitly signals condemnation of exploitative publishing practices and conveys the seriousness of their questionable and unethical practices. This clarity can help to deter authors and institutions from engaging with such outlets, while also holding deceptive publishers publicly accountable. The legal resistance such bad actors have shown to this label demonstrates its power and perceived legitimacy. However, adopting a more neutral term such as “non-recommended” carries the risk of diluting this moral and communicative force. By softening the language, there is a possibility that the actual harm caused by deceptive journals or publishers could be understated, which might inadvertently normalize their existence or reduce researchers’ vigilance or concerns. In this sense, the shift could serve the interests of questionable publishers more than those of the scholarly community. While the proposed terminology may indeed mitigate legal and professional conflicts, its broader impact on awareness, deterrence, and public perception would need to be carefully monitored and empirically assessed.
Conclusion and Future Perspectives
The term "predatory" in academic publishing has generated persistent legal, ethical, professional, and conceptual concerns, prompting a proposed shift to the more neutral description of "non-recommended" journals and publishers. This proposed shift in terminology avoids direct accusations while still emphasizing the need for caution. Beyond its legal advantages, it functions as a form of pragmatic reframing, softening evaluative language, lowering social costs, and promoting a more inclusive and constructive dialogue. Its implicit contrast (“non-recommended” versus “recommended”) invites critical reflection on the criteria for quality publishing and maintains a degree of strategic ambiguity, which can foster broader engagement from stakeholders.
To maximize its effectiveness, future efforts should focus on developing transparent, consensus-based criteria for classifying non-recommended journals and linking these to curated “whitelists” of trustworthy venues (e.g., DOAJ). Adoption of this terminology can enable researchers, institutions, and policymakers to evaluate journals more objectively, address low-quality or deceptive publishing practices, and minimize the risk of legal disputes, reputational harm, or professional conflicts.
Implementation will require global consensus among academic institutions, publishers, indexing bodies, and policymakers, as resistance may arise due to the entrenched use of “predatory” in literature, teaching, and policy documents. Clear, measurable guidelines and internationally recognized frameworks, developed in collaboration with bodies such as COPE, ICMJE, and EASE, will be critical for consistent application.
Future research should empirically test whether adopting “non-recommended” measurably reduces litigation risks, promotes ethical publishing practices, and improves researchers’ ability to evaluate journals. Comparative analyses across disciplines and regions would help to clarify how this linguistic shift affects scholarly communication in practice.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: FMT, DR, BS, BZ, AC, SP, MM, ADC, ANP, SF, KS, PRB, SK, RRS, KB, MC, MN, BG, KK, PP, BSK, GD, MD, BDO, MVB, ARS, UMS, VMG, KNR, AA, AS, PA, JN, JAS, SR, KO, KN, SA, AC, CC, MAR, HAH, and HD were a major contributor to the conception of the study, voting for the items. RQS and AMM were Involved in the literature review, the writing of the manuscript, and data analysis and interpretation. RQS and AMM Confirmation of the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
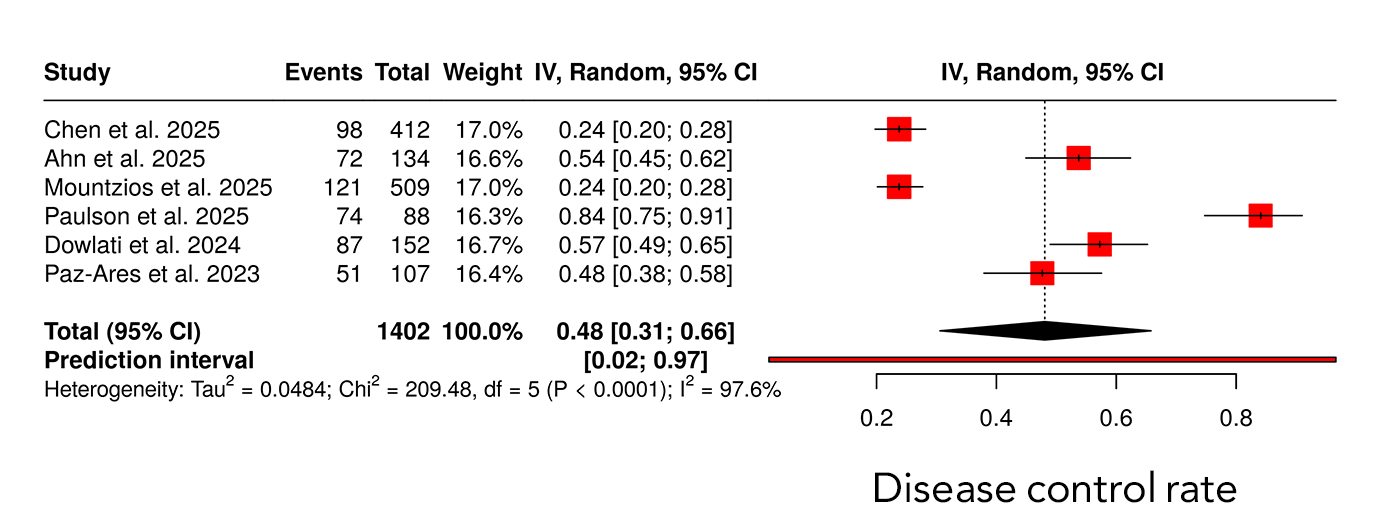
Small Cell Lung Cancer and Tarlatamab: A Meta-Analysis of Clinical Trials
Rebaz M. Ali, Sadraldin A. Braim, Bilal T. Mohammed, Shiraz K. Mohammed, Maria A. Rasool, Shadan...
Abstract
Introduction
Tarlatamab is a Delta-like ligand 3 (DLL3) -directed bispecific T-cell engager recently approved for use in patients with advanced small cell lung cancer (SCLC) after progression on platinum-based therapy. This meta-analysis evaluated the efficacy and safety of tarlatamab as monotherapy and in combination regimens in the treatment of SCLC.
Methods
A systematic review and meta-analysis were conducted in accordance with PRISMA 2020 guidelines. PubMed/MEDLINE and EMBASE were searched from inception through September 2025 to identify clinical trials evaluating tarlatamab in SCLC. Eligible studies reported quantifiable efficacy and/or safety outcomes. Random-effects models were used to pool objective response rate (ORR) and disease control rate (DCR), and Kaplan–Meier methods were applied to assess survival outcomes.
Results
Seven clinical trials involving 1,247 patients with advanced SCLC were included. The pooled ORR was 0.42 (95% CI 0.31–0.54), with response rates ranging from 21–47% in monotherapy studies and up to 48% in combination regimens. Across six studies, pooled DCR was 0.48 (95% CI 0.31–0.66), with DCR reaching up to 87% in combination settings. Median progression-free survival ranged from 3.5 to 5.6 months, while median overall survival ranged from 13.2 to 25.3 months. Pooled time-to-event analyses demonstrated significant reductions in the risk of disease progression and death. Grade 3 and grade 4 adverse events occurred in 5.4% and 1.4% of patients, respectively, although safety reporting was incomplete in several studies.
Conclusion
Tarlatamab demonstrates clinically meaningful antitumor activity with an acceptable safety profile in heavily pretreated SCLC. These findings support DLL3-targeted therapy as a promising treatment strategy and warrant further prospective studies to define its optimal role in the evolving SCLC treatment landscape.
Introduction
Small cell lung cancer (SCLC) is one of the most aggressive and fatal lung malignancies, accounting for approximately 13% to 17% of all lung cancer cases [1]. It is characterized by rapid tumor growth, early dissemination, and a strong tendency toward therapeutic resistance, which collectively complicate diagnosis and management [1,2]. For patients with limited stage disease, standard treatment consists of a combined modality approach using platinum-based chemotherapy, most commonly cisplatin or carboplatin with etoposide, administered concurrently with thoracic radiotherapy. In patients who achieve complete remission, prophylactic cranial irradiation is commonly used to reduce the risk of central nervous system metastases [3,4].
In extensive stage SCLC, treatment strategies have expanded to include immunotherapy, particularly Programmed Death-Ligand 1 (PD-L1) inhibitors, in combination with conventional chemotherapy [4,5]. Despite these advances, long-term survival remains poor due to rapid development of drug resistance, frequent relapse, and substantial treatment-related toxicity [1,6]. Although immune checkpoint inhibitors (ICIs) have transformed outcomes in several malignancies, their benefit in SCLC has been limited. While the high tumor mutational burden of SCLC suggests potential sensitivity to immunotherapy, only a subset of patients derive meaningful benefit from adding ICIs to first-line chemotherapy [7,8]. This limited efficacy has been attributed to factors such as reduced expression of major histocompatibility complex (MHC) molecules, impaired antigen presentation, and marked intratumoral heterogeneity [9,10]. Nevertheless, large international trials have demonstrated improved survival with the addition of PD-L1 inhibitors, including durvalumab, to chemotherapy, supporting the role of chemoimmunotherapy in SCLC [11]. Early evidence suggests that patients with more immunogenic tumors may be the primary beneficiaries of these combination approaches [1,4].
Given the limitations of current therapies, there is a clear need for novel treatment strategies in SCLC. Advances in molecular characterization and understanding of SCLC biology have enabled the development of targeted therapies designed to overcome disease progression and treatment resistance, advancing the potential for personalized therapeutic approaches [12,13]. Tarlatamab is a bispecific T cell engager (BiTE) immunotherapy that targets delta-like ligand 3 (DLL3) on tumor
cells and the Cluster of Differentiation 3 (CD3) receptor on T cells, resulting in T cell activation, cytokine release, and selective cytotoxicity against DLL3-expressing cancer cells. Based on durable antitumor activity and a manageable safety profile observed in the DeLLphi-301 phase 2 trial, tarlatamab received accelerated approval from the United States Food and Drug Administration in May 2024 for patients with extensive stage SCLC who experienced disease progression after platinum-based chemotherapy [14]. This meta-analysis evaluates the efficacy and safety of tarlatamab as monotherapy and in combination with other therapies in the management of SCLC.
Methods
Study design and setting
This systematic review and meta-analysis synthesized evidence from clinical trials assessing the therapeutic efficacy of tarlatamab in SCLC management. Treatment approaches were categorized into four arms: Group A comprising tarlatamab monotherapy, Group B combining tarlatamab with chemotherapy, Group C combining tarlatamab with the PD-L1 inhibitor atezolizumab, and Group D combining tarlatamab with the PD-L1 inhibitor durvalumab. All methodology and reporting followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines.
Data sources and search strategy
A comprehensive search was conducted across PubMed/MEDLINE and EMBASE from inception through September 2025 to identify clinical trials and observational studies evaluating tarlatamab in SCLC. The search strategy combined controlled vocabulary (MeSH terms) and free-text keywords using Boolean operators: (lung OR pulmonary OR bronchi* OR chest OR pleural OR alveol*) AND (tarlatamab OR DeLLphi-300 OR DLL3 OR "delta-like ligand 3" OR Imdelltra OR AMG 757) AND (cancer OR carcinoma OR malignancy OR metastasis). Truncation operators and wildcard searches were used to maximize sensitivity. No language restrictions were applied. Database searches were supplemented by hand-searching relevant journal articles and clinical trial registries to identify additional studies.
Inclusion criteria
Studies were eligible for inclusion if they: (1) evaluated tarlatamab as monotherapy or in combination regimens; (2) reported quantifiable clinical outcomes including response rate, overall survival (OS), progression-free survival (PFS), or time to progression; and (3) enrolled patients with extensive-stage or limited-stage who had received prior platinum-based chemotherapy or other systemic treatment. Studies evaluating tarlatamab as second-line, third-line, or subsequent-line therapy were eligible for inclusion.
Exclusion criteria
Studies were excluded if they: (1) were not clinical trials; (2) did not focus on tarlatamab treatment for SCLC; (3) presented overlapping or duplicate patient populations; (4) lacked adequate efficacy or safety data; (5) were published in languages other than English; (6) were preprints, abstract presentations only, or published in predatory journals.
Study selection process
Two independent researchers screened the titles and abstracts of all identified studies against pre-established inclusion and exclusion criteria. Retrieved articles that appeared potentially eligible underwent full-text review by both reviewers. Any disagreement regarding study eligibility was resolved through discussion and consensus.
Data items
Data extracted from eligible studies included: (1) study and patient characteristics (first author name, year of publication, sample size, trial phase); (2) demographic variables (median age, gender distribution, smoking status); (3) clinical baseline characteristics (ECOG performance status, metastatic sites, prior platinum-based chemotherapy, number of prior treatment lines, prior immunotherapy exposure, prior radiotherapy); (4) treatment details (tarlatamab dosing, administration frequency, median duration of therapy); (5) adverse event data (graded according to Common Terminology Criteria for Adverse Events [CTCAE], including Grade 1-5 events and serious adverse events); and (6) clinical efficacy outcomes (response rates, disease control rate, PFS, and OS).
Data analysis and synthesis
Data were extracted and organized using Microsoft Excel (2019). Descriptive statistical analyses were performed using Statistical Package for Social Sciences (SPSS) version 26.0, with results summarized as frequencies, percentages, medians, and ranges. Meta-analyses were conducted to synthesize efficacy outcomes. For binary outcomes (objective response rate and disease control rate), forest plots were generated using METAANALYSISONLINE to visualize pooled effect estimates and heterogeneity. For time-to-event outcomes (PFS and OS), Kaplan-Meier methods were used to estimate median survival and percentiles with 95% confidence intervals (CI) calculated using the Brookmeyer and Crowley method [15].
Results
Study selection
A systematic search of peer-reviewed databases initially identified 214 potentially relevant records related to tarlatamab and small cell lung cancer. Following removal of duplicates (n=2), non-English publications (n=2), and records with abstract-only availability (n=9), 201 studies underwent title and abstract screening. This process identified 188 studies for comprehensive full-text review. Application of pre-established eligibility criteria resulted in 14 studies selected for detailed evaluation. Of these, seven studies were subsequently excluded due to preprint status (n=4) and publication in predatory journals (n=3), resulting in a final cohort of 7 eligible clinical trials included in the meta-analytic synthesis [16-22]. The PRISMA flow diagram illustrating the complete study selection process is presented in (Figure 1). All included references reviewed to exclude non-peer-reviewed data [23].
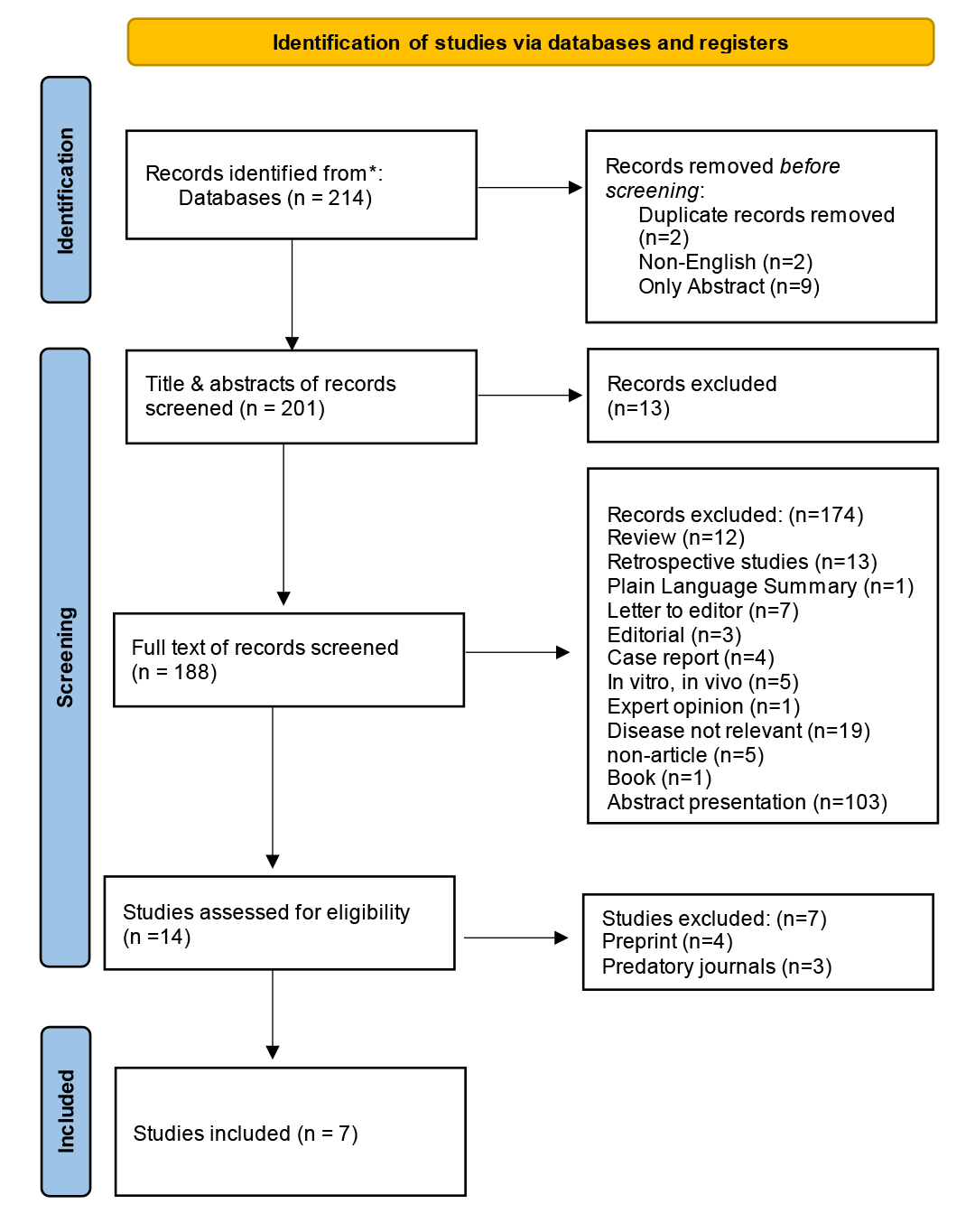
Patient characteristics
Across the seven included clinical trials, baseline study-level characteristics indicated relatively homogeneous populations of patients with advanced small cell lung cancer. Most studies were early-phase trials evaluating tarlatamab as monotherapy or in combination regimens. Trial-level median ages ranged from 62 to 65 years, with male patients consistently representing the majority across individual studies. Brain and liver metastases were identified as the most commonly reported sites of metastatic disease across all trials (Table 1).
|
Author |
Year of publication |
Type of therapy |
Phase of clinical trial |
No. of patients |
Gender |
Median Age |
Smoking Status |
Metastasis |
||||
|
Male |
Female |
Current |
Never |
Former |
N/A |
|||||||
|
Chen et al [16] |
2025 |
Tarlatamab |
1&2 |
412 |
263 |
149 |
63 |
66 |
32 |
312 |
2 |
Brain, liver |
|
Hummel H-D et al [18] |
2025 |
Tarlatamab |
2 |
100 |
72 |
28 |
N/A |
N/A |
N/A |
N/A |
100 |
Brain, liver |
|
Ahn et al [19] |
2025 |
Tarlatamab |
2 |
134 |
96 |
38 |
65 |
24 |
9 |
101 |
0 |
Brain, liver |
|
Mountzios et al [20] |
2025 |
Tarlatamab + Chemotherapy |
3 |
509 |
182 |
72 |
64 |
54 |
23 |
177 |
0 |
Brain, liver |
|
Paulson et al [21] |
2025 |
Tarlatamab + Atezolizumab, Tarlatamab + durvalumab
|
1b |
88 |
55 |
33 |
64 |
21 |
4 |
63 |
0 |
Brain, liver |
|
Dowlati et al [17] |
2024 |
Tarlatamab |
1 |
152 |
85 |
67 |
62 |
28 |
13 |
111 |
0 |
Brain, liver |
|
Paz-Ares et al [22] |
2023 |
Tarlatamab |
1 |
107 |
61 |
46 |
63 |
14 |
10 |
81 |
2 |
Brain, liver |
|
N/A: Not applicable |
||||||||||||
A total of 1,247 patients were included in the analysis. The overall median age was 63.5 years. Male patients accounted for 814 cases (65.3%), while 433 patients (34.7%) were female. Smoking status was reported for most patients, with former smokers constituting the largest subgroup (845 patients, 67.8%), followed by current smokers (207 patients, 16.6%) and never smokers (91 patients, 7.3%). Smoking history was not reported in 104 patients (8.3%) Consistent with the trial-level findings, metastatic involvement most commonly affected the brain and liver (Table 2).
|
Variables |
Number (%) |
|
Age (Year), (Median, IQR) |
63.5 (63–64) |
|
Sex -Male -Female |
-814 (65.3%) -433 (34.7%) |
|
Smoking status - Current - Never - Former - Not mentioned |
- 207 (16.6%) -91 (7.3%) -845 (67.8%) -104 (8.3%) |
|
ECOG status -ECOG status (0) -ECOG status (1) -ECOG status (2) -Not mentioned
|
Total number (1247) -280 (22.5%) -550 (44.1%) -5 (0.4%) -412 (33.0%)
|
|
Metastasis -Brain Yes No -Liver Yes No
|
Total number (1247)
-1247 (100%) -0 (0%)
-1247 (100%) -0 (0%) |
|
Previously prior therapy |
|
|
Prior platinum‑based chemotherapy/regimen - Yes - No |
Total number (1086) - 1086 (100%) - 0 (0.0) |
|
Prior PD‑1/PD‑L1 therapy - Yes - No - Not mentioned |
Total number (1502) - 963 (64.1%) - 439 (29.2%) - 100 (6.7%) |
|
Prior radiotherapy - Yes - No - Not mentioned |
Total number (1502) - 201 (13.4%) - 58 (3.9%) - 1243 (82.7%) |
|
Number of prior lines of systemic therapy - 1 line - 2 lines - 3 lines |
Total number (1400) - 728 (52.0%) - 413 (29.5%) - 259 (18.5%) |
|
Treatment group - Tarlatamab alone - Combination group |
Total number (1247) -1159 (93%) -88 (7%) |
|
Response to Tarlatamab alone and combination group - Complete response - Partial response - Stable disease - Progressive disease - Not mentioned |
Number of Patients (1247) - 16 (1.3%) - 216 (17.3%) - 207 (16.6%) - 142 (11.4%) - 666 (53.4%) |
|
Objective response rate (range %) |
21-48 |
|
Disease control rate (range %) |
51-87 |
|
Overall survival (Months) (Median, IQR) |
14.3 (13.2-19.0) |
|
Progression free survival (Months) (Median, IQR) |
4.9 (3.7-5.4) |
| IQR: Interquartile range, ECOG: Eastern Cooperative Oncology Group | |
Prior Therapy and Performance Status
All patients had received prior platinum-based chemotherapy. Most patients had undergone one prior line of systemic therapy (728 patients, 52.0%), followed by two prior lines in 413 patients (29.5%) and three prior lines in 259 patients (18.5%). Previous exposure to PD-1 or PD-L1 inhibitor therapy was reported in 963 patients (64.1%), while 439 patients (29.2%) had not received prior immunotherapy. A history of prior radiotherapy was documented in 201 patients (13.4%). Among patients with reported Eastern Cooperative Oncology Group (ECOG) performance status, 280 patients (22.5%) had an ECOG score of 0, 550 patients (44.1%) had a score of 1, and 5 patients (0.4%) had a score of 2. ECOG performance status was not reported for 412 patients (33.0%) (Table 2 & 3).
|
Author
|
No. of patients |
Number of prior lines of systemic therapy |
Prior platinum‑based chemotherapy/regimen |
Prior PD‑1/PD‑L1 therapy |
Prior radiotherapy |
||||||||
|
Median |
1 line |
2 lines |
3 lines |
Yes |
No |
Yes |
No |
N/A |
Yes |
No |
N/A |
||
|
Chen et al [16] |
412 |
N/A |
55 |
219 |
138 |
412 |
0 |
276 |
136 |
0 |
0 |
0 |
412 |
|
Hummel et al [18] |
100 |
2 |
N/A |
N/A |
N/A |
100 |
0 |
0 |
0 |
100 |
0 |
0 |
100 |
|
Ahn et al [19] |
134 |
2 |
2 |
87 |
45 |
134 |
0 |
101 |
33 |
0 |
0 |
0 |
134 |
|
Mountzios et al [20] |
254 |
N/A |
509 |
0 |
0 |
509 |
0 |
360 |
149 |
0 |
0 |
0 |
509 |
|
Paulson et al [21] |
88 |
N/A |
88 |
0 |
0 |
88 |
0 |
77 |
11 |
0 |
0 |
0 |
88 |
|
Dowlati et al [17] |
152 |
2 |
44 |
62 |
44 |
152 |
0 |
96 |
56 |
0 |
116 |
36 |
0 |
|
Paz-Ares et al [22] |
107 |
2 |
30 |
45 |
32 |
103 |
0 |
53 |
54 |
0 |
85 |
22 |
0 |
Efficacy outcomes
Across the seven included studies, disease response was assessed using standardized Response Evaluation Criteria in Solid Tumors (RECIST) or investigator-defined criteria. In the evaluable population (n=1,247), complete response was observed in 16 patients (1.3%), partial response in 216 patients (17.3%), stable disease in 207 patients (16.6%), and progressive disease in 142 patients (11.4%). Response status was not reported or evaluable in 666 patients (53.4%), primarily due to early study termination, lack of post-baseline imaging, or classification as non-evaluable per trial protocols (Table 2).
In studies evaluating tarlatamab monotherapy, disease control rates (DCR) ranged from 51% to 82%, with objective response rates (ORR) ranging from 21% to 47%. In studies assessing combination therapy, higher response rates were observed, with DCR reaching up to 87% and ORR up to 48%. Median PFS reported across studies ranged from 3.5 to 5.6 months, while median OS ranged from 13.2 to 25.3 months. In the Asian subgroup, median OS reached 19.0 months, with a median PFS of 5.4 months (Table 4).
|
Partial response |
Complete response |
Stable disease |
Progressive Disease |
Not mentioned |
Disease control rate |
Objective response rate |
Median overall survival (months) |
Median progression free survival (months) |
|
N/A |
N/A |
N/A |
N/A |
0 |
82 |
47 |
5.8 |
3.7 |
|
N/A |
N/A |
N/A |
N/A |
0 |
N/A |
40 |
14.3 |
4.9 |
|
54 |
5 |
44 |
24 |
14 |
75.25 |
43.15 |
19 (Asian group) |
5.4 (Asian group) |
|
86 |
3 |
84 |
56 |
25 |
173 |
35 |
13.6 |
5.3 |
|
19 |
2 |
N/A |
N/A |
0 |
53 |
21 |
25.3 |
5.6 |
|
34 |
4 |
49 |
53 |
12 |
87 |
25 |
17.5 |
3.5 |
|
23 |
2 |
30 |
9 |
0 |
51 |
48 |
13.2 |
3.7 |
The forest plot displays individual study estimates of objective response rate with corresponding 95% confidence intervals (CIs), pooled using a random-effects model. The size of each square represents the weight of the study in the meta-analysis, while horizontal lines indicate 95% CIs. The diamond represents the pooled ORR with its 95% CI. A prediction interval is shown to reflect the expected range of treatment effects in future studies. Significant heterogeneity was observed across studies (I² = 94.6%, P < 0.0001) (Figure 2).
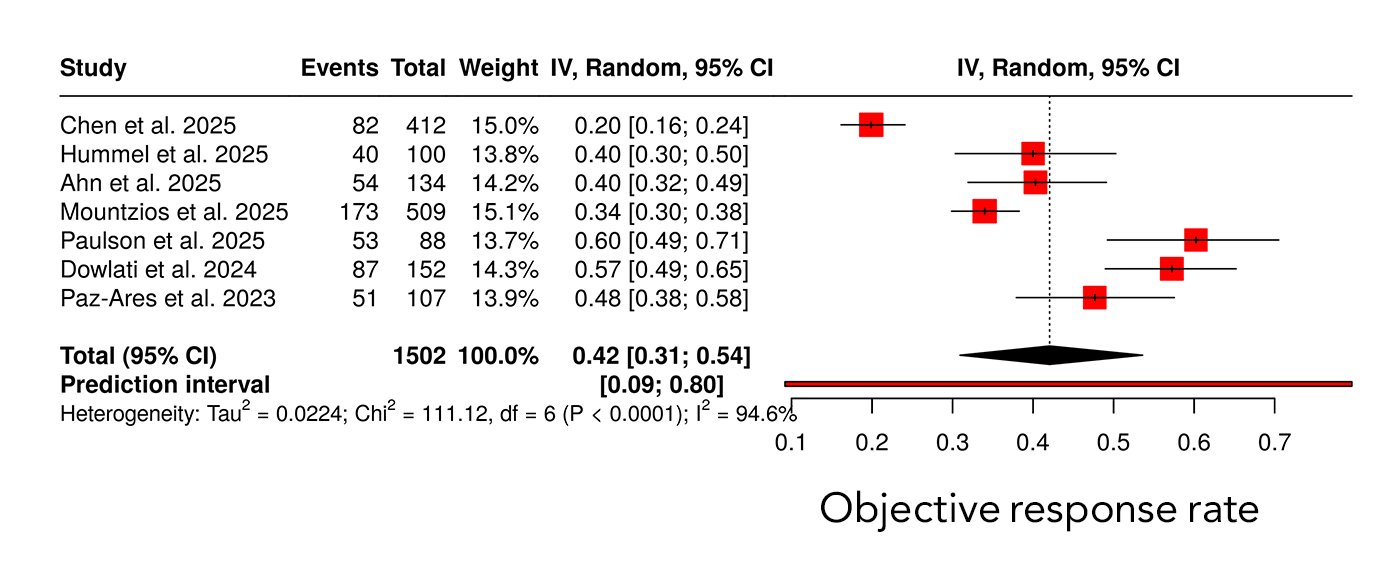
Across six studies including 1402 patients, the pooled DCR estimate was 0.48 (95% CI 0.31–0.66). Substantial heterogeneity was present, with an I² value of 97.6%. The prediction interval ranged from 0.02 to 0.97, reflecting wide variability in disease control outcomes across studies (Figure 3). Median PFS differed across studies, with reported median values ranging between approximately 3 and 6 months. The pooled Kaplan–Meier analysis demonstrated a hazard ratio of 0.76 with a statistically significant p-value of 0.003, as shown in the figure. Median OS across studies ranged from approximately 13 to 25 months. The pooled Kaplan–Meier analysis demonstrated a hazard ratio of 0.71 with a p-value of 0.012. Differences in survival trajectories among individual studies were observed over extended follow-up durations of up to 40 months. (Figure 4).
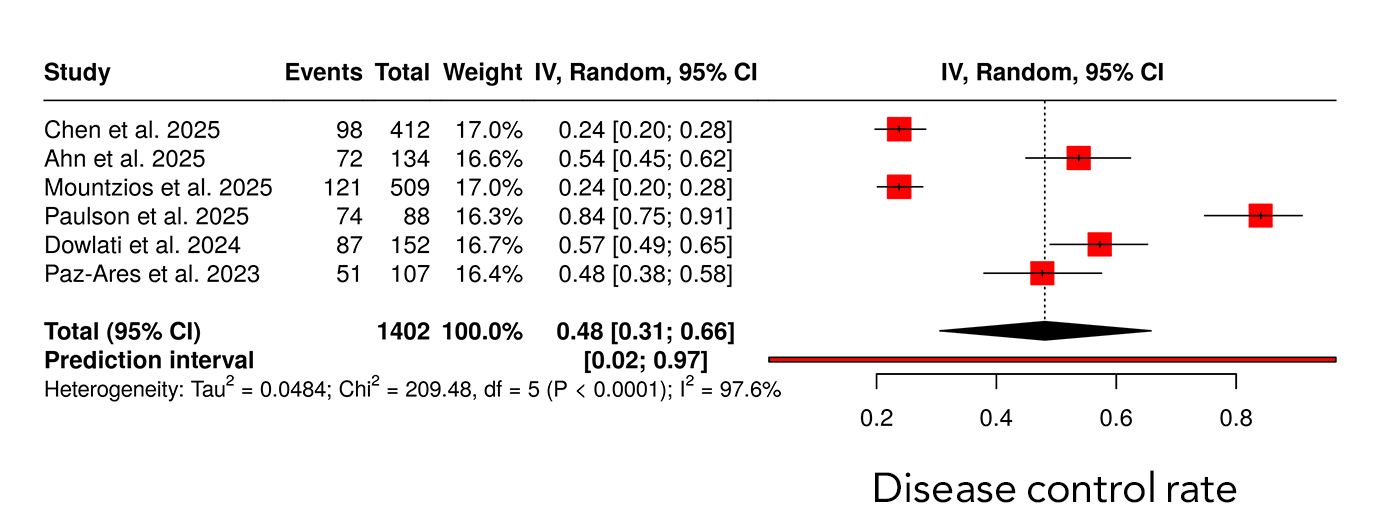
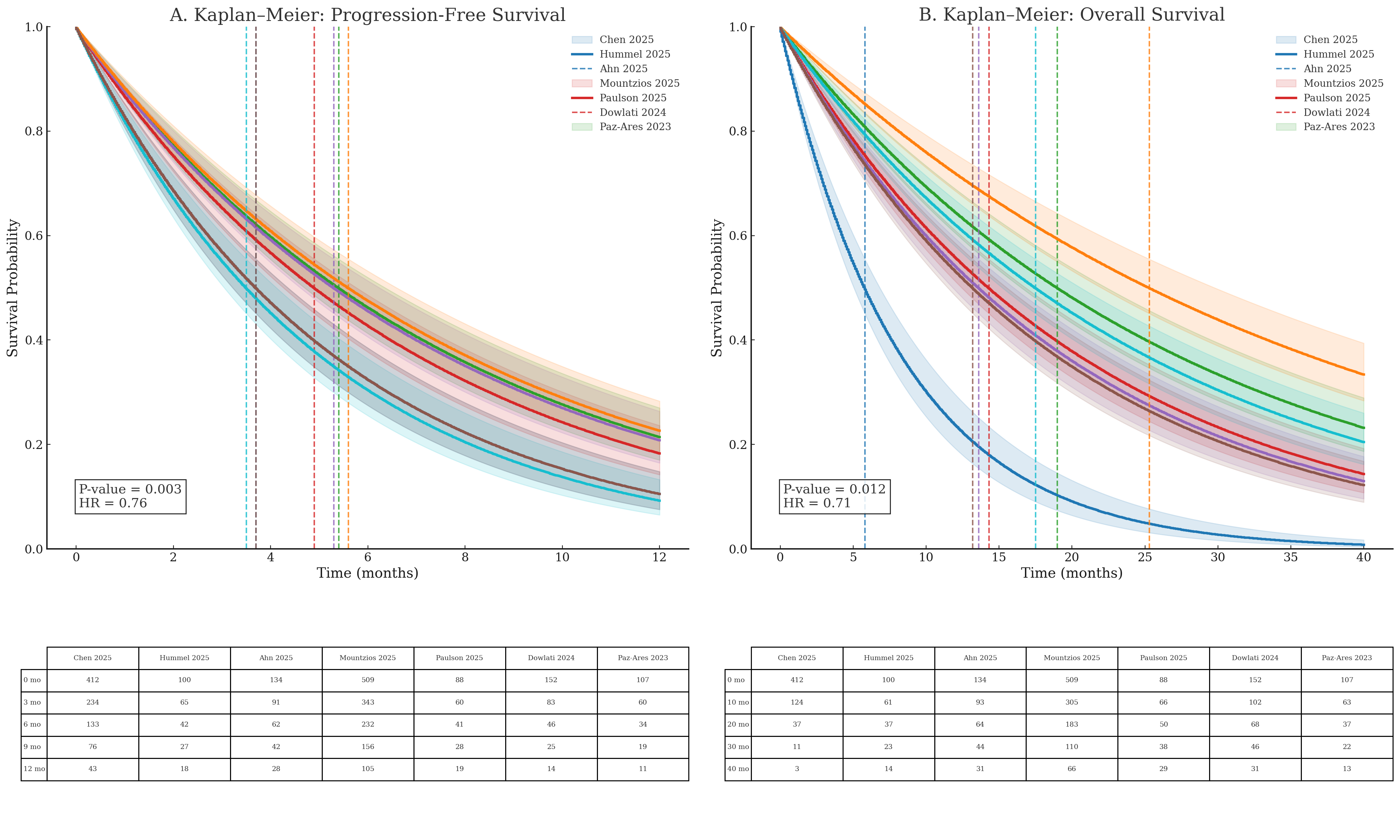
Safety outcomes
Among the reported populations, grade 2 or higher adverse events occurred in 136 patients, while grade 3 adverse events were reported in 68 patients (5.4%) and grade 4 adverse events in 17 patients (1.4%). In studies where subgroup-specific data were available, including patients from Asian populations, grade 2 or higher adverse events were reported in 39 patients, grade 3 adverse events in 24 patients, grade 4 adverse events in 7 patients, and grade 5 adverse events in 2 patients. Serious adverse events were reported in 129 patients in studies that documented this outcome. For a substantial proportion of patients, adverse event severity and grading were not reported and were therefore categorized as not available in the safety dataset (Table 5).
|
Adverse Event Rates |
|||||
|
Grade 1 |
Grade ≥ 2 |
Grade ≥ 3 |
Grade ≥ 4 |
Grade ≥ 5 |
Serious adverse events |
|
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
N/A |
39 (Asian group) * |
24 (Asian group) * |
7 (Asian group) * |
NA |
2 (Asian group) * |
|
N/A |
N/A |
136 |
N/A |
N/A |
129 |
|
8 |
29 |
38 |
13 |
N/A |
N/A |
|
N/A |
119 |
68 |
17 |
N/A |
65 |
|
N/A |
N/A |
33 |
N/A |
1 |
55 |
|
* Were available only for the Asian subgroup, as corresponding safety data for the overall study population were not reported. |
|||||
Discussion
Small cell lung cancer remains one of the most aggressive solid malignancies, characterized by rapid tumor proliferation, early metastatic dissemination, and persistently poor survival outcomes. Despite advances in chemo-immunotherapy, the disease is largely incurable in advanced stages, with most patients relapsing within six months following first-line platinum-based chemotherapy. Outcomes after platinum failure are particularly poor, as standard second-line therapies such as topotecan and lurbinectedin demonstrate ORR below 20% and median OS rarely exceeding 10 months [22,24]. These limited clinical benefits underscore a major unmet therapeutic need in relapsed SCLC. Consequently, there has been growing interest in identifying biologically driven targets capable of delivering more durable responses with acceptable safety profiles [20,24]. Among emerging targets, DLL3 has gained attention due to its high and selective expression on SCLC tumor cells and minimal presence in normal tissues [1,20,25]. This tumor-restricted expression pattern provides a strong biological rationale for DLL3-directed therapeutic strategies in relapsed SCLC.
Previous prospective and real-world studies have consistently demonstrated poor outcomes for patients with relapsed small cell lung cancer following platinum failure. Rudin et al. and Paz-Ares et al. reported objective response rates generally below 20% with standard second-line therapies such as topotecan and lurbinectedin, with median overall survival rarely exceeding 10 months [22,24]. These findings highlight a substantial unmet therapeutic need in this setting [24]. In contrast, the present meta-analysis demonstrated enhanced clinical activity with tarlatamab-based therapy, yielding a pooled objective response rate of 42% and disease control rates ranging from 51% to 87%, depending on the treatment regimen. Notably, both monotherapy and combination approaches achieved tumor response rates that exceeded those historically reported with conventional chemotherapy. Although cross-study comparisons should be interpreted with caution, the magnitude of improvement observed relative to prior literature supports the therapeutic potential of DLL3-targeted therapy with tarlatamab in relapsed SCLC and underscores the need for further prospective validation.
Metastatic burden at baseline was substantial and consistent with advanced small cell lung cancer. Previous studies have shown that the brain and liver are among the most frequent sites of metastasis in SCLC, with brain involvement reported in approximately 40–70% of patients over the disease course[20,22]. In line with these reports, brain and liver metastases were the most commonly observed metastatic sites across the included trials in the present meta-analysis. The presence of extensive metastatic disease reflects a real-world advanced SCLC population and reinforces the external validity and generalizability of the observed treatment outcomes.
Performance status is a well-established prognostic factor in small cell lung cancer; however, its influence on treatment outcomes remains a subject of debate, particularly in the context of clinical trial selection. Rudin et al. and Paz-Ares et al. have shown that patients with impaired ECOG performance status experience lower response rates, shorter progression-free survival, and reduced overall survival, yet these patients are frequently underrepresented in prospective trials [20,22]. In the present meta-analysis, most evaluable patients had favorable baseline performance status, with 22.5% classified as ECOG 0 and 44.1% as ECOG 1, indicating that approximately two-thirds of patients entered treatment with preserved functional capacity. Only a small proportion of patients had an ECOG score of 2 (0.4%), while ECOG status was not reported in one-third of cases. This imbalance highlights an ongoing controversy regarding the generalizability of trial-based efficacy estimates and suggests that treatment benefits may be overestimated when extrapolated to patients with poorer functional reserve.
Historically, patients with relapsed small cell lung cancer have experienced poor outcomes following platinum failure. Rudin et al., Paz-Ares et al., Dowlati et al., and Sands et al. have reported that standard second-line therapies such as topotecan and lurbinectedin typically achieve objective response rates below 20%, with median overall survival rarely exceeding 10 months [14,17,22,24]. Against this background, the present meta-analysis demonstrates that tarlatamab provides clinically meaningful antitumor activity in a heavily pretreated SCLC population. Among 1,247 evaluable patients, complete and partial responses were observed in 1.3% and 17.3% of patients, respectively, resulting in a pooled objective response rate of 42%. Furthermore, disease control was achieved in nearly half of treated patients, with rates reaching up to 87% in combination regimens, substantially exceeding historical chemotherapy benchmarks.
Survival outcomes further support the clinical relevance of these findings. Median progression-free survival ranged from 3.5 to 5.6 months, while median overall survival extended from 13.2 to 25.3 months across individual studies. Rudin et al., Paz-Ares et al., Dowlati et al., and Sands et al. have consistently reported that historical second-line therapies such as topotecan, lurbinectedin, and amrubicin yield median overall survival of approximately 5.8–10 months, underscoring the limited durability of benefit in this setting [14,17,22,24]. Against this benchmark, the observed 2–3-fold improvement in median overall survival with tarlatamab suggests a clinically meaningful survival advantage in relapsed SCLC. Pooled time-to-event analyses further demonstrated statistically significant reductions in the risk of disease progression and death, with hazard ratios of 0.76 for progression-free survival and 0.71 for overall survival. Notably, subgroup analyses indicated that Asian patients achieved a median overall survival of approximately 19 months, consistent with individual trial reports and supporting the reproducibility of benefit across populations. Remarkably, survival outcomes achieved with tarlatamab in the relapsed setting approached or in some studies exceeded those reported with first-line chemo-immunotherapy regimens, such as CASPIAN and KEYNOTE-604, where median overall survival ranges from 12.3 to 13.0 months [17,22,24]. Although cross-trial comparisons should be interpreted with caution, achieving comparable survival outcomes in later treatment lines represents a particularly striking observation, given the well-established pattern of diminishing benefit with successive therapies in SCLC.
The observed efficacy of tarlatamab is biologically plausible and aligns with the established role of DLL3 in small cell lung cancer pathogenesis. Ding et al. and Zhang et al. have demonstrated that DLL3 is highly expressed in neuroendocrine SCLC and contributes to tumor proliferation and maintenance through dysregulated Notch signaling [1,25]. By simultaneously engaging CD3-positive T cells and DLL3-expressing tumor cells, tarlatamab induces potent T-cell–mediated cytotoxicity independent of major histocompatibility complex class I–restricted antigen presentation, thereby circumventing key immune evasion mechanisms characteristic of SCLC, as highlighted by Rudin et al. and Paz-Ares et al. [22,24]. This mechanism of action clearly distinguishes tarlatamab from immune checkpoint inhibitors and may explain its robust antitumor activity in a disease that has historically demonstrated limited responsiveness to immunotherapy.
Importantly, tarlatamab appears to offer superior efficacy and tolerability compared with earlier DLL3-targeted approaches such as rovalpituzumab tesirine. Rudin et al. and Paz-Ares et al. reported that although rovalpituzumab tesirine demonstrated modest response rates, it failed to improve survival and was associated with substantial toxicity and high treatment discontinuation rates in phase III trials [22,24]. In contrast, tarlatamab exploits endogenous immune effector mechanisms without the delivery of a cytotoxic payload, resulting in an improved therapeutic index and a more favorable safety profile, as supported by findings from Rudin et al., Paz-Ares et al., and Ding et al. [1,22,25].
Across the included studies, tarlatamab was generally well tolerated. Severe adverse events were infrequent, with grade 3 and grade 4 toxicities reported in 5.4% and 1.4% of patients, respectively. The most commonly observed treatment-related adverse events were consistent with the expected profile of T-cell engager therapies, particularly cytokine release syndrome, which was predominantly low-grade and rapidly reversible with standard supportive measures. Paz-Ares et al. and Sands et al. reported that neurotoxicity was uncommon and typically mild, with grade 3–4 events occurring in only a small minority of patients [14,22]. Compared with conventional cytotoxic chemotherapy, these findings suggest a more favorable balance between efficacy and tolerability. This observation was further reinforced by the phase III DeLLphi-304 trial, in which Rudin et al. demonstrated significantly lower rates of severe adverse events and treatment discontinuation with tarlatamab compared with physician’s-choice chemotherapy [22].
Indirect treatment comparisons using real-world data provide additional support for these findings. After adjustment for baseline prognostic factors, Wang et al. reported that tarlatamab was associated with significantly improved overall survival, progression-free survival, and objective response rate compared with real-world comparator therapies [2], suggesting that the observed clinical benefit extends beyond the controlled setting of clinical trials and may be generalizable to broader patient populations. Substantial heterogeneity was observed across pooled analyses, reflecting differences in study design, treatment regimens, and patient characteristics. In the context of relapsed SCLC, such variability is expected and reflects real-world clinical complexity. Importantly, the persistence of tarlatamab activity across heterogeneous settings supports the robustness of its antitumor effect. Patients with lower baseline tumor burden, preserved performance status, and absence of liver metastases appeared more likely to achieve sustained disease control, suggesting that patient selection and earlier intervention may optimize outcomes. These observations should be interpreted cautiously and provide hypothesis-generating insights that warrant further prospective evaluation.
Several limitations should be acknowledged. The meta-analysis included a limited number of studies, many of which were early-phase trials, and substantial heterogeneity was observed across efficacy outcomes. Response assessment was incomplete in a proportion of patients, particularly in dose-escalation studies, and safety reporting was inconsistent across trials. Additionally, the absence of individual patient-level data precluded detailed subgroup and biomarker analyses. These limitations underscore the need for further randomized trials and biomarker-driven studies to refine patient selection and confirm the long-term clinical role of tarlatamab in SCLC.
Conclusion
This meta-analysis revealed the clinical relevance of DLL3-targeted therapy as a promising treatment strategy for advanced small cell lung cancer. The findings indicate that tarlatamab offers meaningful antitumor activity in a setting characterized by limited therapeutic options following standard treatments. Importantly, the favorable balance between efficacy and tolerability supports the continued clinical development of this approach. Further well-designed prospective studies are needed to clarify the optimal positioning of tarlatamab within the evolving treatment landscape of SCLC.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: KKM, SMA and KAN were responsible for data collection and analysis, and final approval of the manuscript. BAA and RML were major contributors to the conception of the study, as well as to the literature search for related studies. MQM, ZKH, SAH, MAR, SKM, BTM, and SAB were involved in the literature review, the design of the study, and the critical revision of the manuscript. BAA was involved in the literature review, the writing of the manuscript, and design of the study and data interpretation. BAA and RML confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Use of AI: ChatGPT version 5.2 (OpenAI) was used solely for language editing, paraphrasing, and improvement of clarity and grammar in this manuscript. The artificial intelligence tool did not contribute to the study design, data collection, data analysis, data interpretation, or the generation of scientific content. All outputs produced with the assistance of ChatGPT were carefully reviewed, verified, and approved by the authors. The authors take full responsibility for the accuracy, integrity, and originality of the entire manuscript.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Case Reports
Early Onset of Coronary Subclavian Steal Syndrome: A Case Report and Literature Review
Jalal Gareeb, Farman J. Ahmed, Hemn H. Mohammad, Karokh F. Hamahussein, Soran H. Tahir, Sumaya...
Abstract
Introduction
Coronary subclavian steal syndrome (CSSS) is a rare phenomenon that often goes undiagnosed and causes severe complications, including death. This report presents a case of CSSS with unexpectedly early presentation following coronary artery bypass grafting (CABG).
Case presentation
A 49-year-old male with diabetes, smoking history, and ischemic heart disease underwent CABG with a left internal mammary artery graft to the LAD and saphenous vein grafts. Three months later, he presented with exertional chest pain and left arm discomfort. Examination revealed a significant inter-arm blood pressure difference (right 140/90 mmHg, left 90/65 mmHg) and a diminished left radial pulse. Computed tomography angiography revealed complete proximal left subclavian artery occlusion with patent grafts. Percutaneous revascularization with balloon pre-dilatation and a 7.0 × 27 mm stent restored flow. The procedure was uneventful, and at 10-month follow-up, he remained asymptomatic with normalized arm pressures.
Literature review
A review of ten recent CSSS cases revealed a predominance of males (7/10), with ages ranging from 58 to 81 years. Comorbidities included cardiovascular, renal, and metabolic disorders. Chest pain was the most frequent presenting symptom. The interval from CABG to CSSS onset ranged from two days to 13 years. Management strategies encompassed percutaneous coronary interventions, nitrates, antihypertensives, and statins, with all patients achieving favorable outcomes.
Conclusion
Coronary subclavian steal syndrome can present shortly after CABG. Percutaneous endovascular stenting via a dual approach might offer good long-term outcomes.
Introduction
Subclavian steal syndrome (SSS) is a condition in which severe stenosis or occlusion of the proximal subclavian artery causes reversal of blood flow in the ipsilateral vertebral artery [1]. It affects 0.6%–6.4% of the general population, with higher prevalence in elderly men owing to the increased atherosclerosis [2]. In patients with three-vessel or left main coronary artery disease, the prevalence of SAS can be as high as 5.3% [1].
Coronary subclavian steal syndrome (CSSS) is an uncommon complication of coronary artery bypass grafting (CABG) that arises when the left internal mammary artery (LIMA) is used in patients with previously unrecognized, significant left subclavian artery stenosis (SAS) [3]. This phenomenon reduces blood flow through the internal mammary artery (IMA), thereby compromising coronary perfusion and potentially resulting in myocardial ischemia and angina [1].
The most common cause of subclavian artery stenosis is atherosclerosis, and, more rarely, arteritis, radiation, fibromuscular dysplasia, and compression syndromes [4]. Using the LIMA as the primary conduit increases the risk of developing CSSS. Although CSSS remains rare, with a reported incidence of 0.2%–6.8%, its occurrence is rising in parallel with the growing use of LIMA in CABG [3]. A limited number of case reports and case series on CCCS have been published in the available literature [4]. CSSS typically manifests several years after CABG [3]. However, this case represents one of the earliest documented occurrences of CSSS associated with complete subclavian artery occlusion, highlighting its potential for unexpectedly early presentation. This report was written following CaReL guidelines, and all the references have been checked for validity [5,6].
Case presentation
Patient information
A 49-year-old male with a history of smoking, long-standing diabetes mellitus, and ischemic heart disease underwent CABG. The surgery involved a left internal mammary artery LIMA graft to the left anterior descending (LAD) artery and saphenous vein grafts to the first and last obtuse marginal branches, as well as the first diagonal branch. Three months later, he presented with recurrent chest pain and left arm discomfort, predominantly triggered by exertion of the left upper limb.
Clinical findings
The patient appeared well and hemodynamically stable. Notably, systemic peripheral arterial blood pressure measured 140/90 mmHg in the right arm and 90/65 mmHg in the left arm, with a diminished radial pulse on the left side.
Diagnostic approach
An electrocardiogram was performed and was unremarkable, and echocardiography revealed no significant abnormalities. A CT angiography of the coronary grafts and thoracic outlet was subsequently obtained, demonstrating complete occlusion of the left subclavian artery just proximal to the LIMA ostium. In contrast, all coronary grafts, including the LIMA, remained patent (Figure 1).
Therapeutic intervention
Percutaneous revascularization of the left subclavian artery (SA) was undertaken via a left radial approach. Selective angiography of the LIMA demonstrated complete occlusion of the proximal SA, with opacification limited to a few centimeters of the proximal LIMA and rapid retrograde filling of the SA, consistent with collateral flow from the LAD artery through the LIMA.
The lesion was crossed using a 0.014" Pilot 200 guidewire (Abbott, USA). Pre-dilatation was performed with a 4.0 × 20 mm semi-compliant balloon, followed by deployment of a 7.0 × 27 mm balloon-expandable peripheral stent (Medtronic, USA). Optimal stent positioning was ensured with angiography via the right femoral approach. Post-procedural imaging confirmed restoration of anterograde flow, with complete resolution of the SA occlusion and improved perfusion of both the LIMA and distal SA (Figure 2). Hemostasis at the radial access site was achieved using a compression device, and manual compression was applied at the femoral site until adequate hemostasis was confirmed. No vascular closure device or surgical drain was equired. The patient remained hemodynamically stable throughout the procedure, with no immediate complications.
This intervention eliminated coronary subclavian steal, thereby preserving LIMA graft patency and ensuring adequate myocardial perfusion. He was discharged on dual antiplatelet therapy along with standard medical management.
Follow-up
Ten months post-intervention, he remained asymptomatic, with no recurrence of chest pain or exertional discomfort. Blood pressure measurements and pulse volume were comparable between the right and left arms.
Discussion
The LIMA is the preferred and most frequently used conduit for myocardial revascularization. During CABG, the proximal end of the LIMA is typically attached to the left SA, while the distal end is anastomosed to the stenotic coronary artery [7]. Anatomically, the LIMA gives off several side branches that supply the chest wall, sternum, and adjacent structures. During bypass surgery, these branches are typically ligated to prevent coronary steal from the LAD, which could otherwise lead to cardiac ischemia [7].
The clinical presentation of CSSS is highly variable; it can manifest as stable angina, STEMI, arrhythmias, or sudden death, resembling other coronary syndromes [1]. Most patients develop stable angina years after CABG (mean 9 ± 8.4 years). However, Mustapić et al. described STEMI on the second postoperative day due to missed SA occlusion [3]. The mean duration from CABG to clinical presentation of CSSS was 4.3±4.7 years, with some durations exceeding 10 years; chest pain was the most common symptom, followed by dyspnea (Table 1) [1,3,4,7-11]. In contrast, this patient developed subacute angina and left arm discomfort three months after CABG, and progressed to complete occlusion, earlier than the mean interval. The absence of ECG changes or hemodynamic changes reflects a more indolent yet clinically significant presentation.
|
Author, year [reference] |
Cases |
Age |
Sex |
Comorbidities |
Presentation |
Period from CABG to presentation |
ECG |
Management |
Outcome |
Follow-up |
|
Carmona et al., 2022 [4] |
1 |
71 |
M |
IHD, CKD, COPD, HT, dyslipidemia & T2D |
Chest pain, abdominal pain & oliguria |
13 years |
ST depression (V5, V6, aVL), ST elevation in aVR |
Coronary angiography, Angioplasty of the middle LCX with stents, Angioplasty and stenting of the LSA, & Aspirin + Clopidogrel |
LVEF improved from 20% to 40%
|
N/A |
|
Multani et al., 2025 [8] |
1 |
64 |
M |
CAD |
Recurrent angina and dyspnea |
2 years |
No ischemic changes |
Beta-blocker, nitrate, ranolazine, Percutaneous occlusion of large unligated LIMA side branch using vascular plug |
Improved LAD flow & resolution of angina |
6 months |
|
Trebišovský et al., 2025 [9] |
1 |
58 |
M |
IHD, HT, hemochromatosis |
Exertional chest pain & dyspnea |
8 months |
No ischemic changes |
Percutaneous obliteration of LCA using Amplatz Vascular Plug II |
Resolution of exertional angina |
1 year |
|
Elhakim et al., 2025 [10] |
1 |
81 |
M |
IHD, Hyperlipoproteinemia & HT |
Cardiogenic shock, STEMI of the anterior lateral wall |
9 years |
Sinus rhythm, ST elevation in leads V1–V6, I, and aVL
|
Emergent cardiac catheterization, PCI of the LSA using two stents, Aspirin, Clopidogrel, Statin, ACE inhibitor, Beta-blocker |
Hemodynamic stabilization & symptom relief |
1 year |
|
Şahin et al., 2021 [1] |
1 |
77 |
F |
N/A |
Exertional dyspnea, chest pain & exhaustion |
10 years |
Sinus rhythm, no ischemic changes |
Coronary angiography, Percutaneous transluminal angioplasty with drug-coated balloon, self-expandable stent placement, aspirin & clopidogrel. |
Correction of reverse flow in LIMA, resolution of angina |
1 year |
|
Choubdar, 2024 [11] |
1 |
60 |
F |
CAD, PAD, CVA, T1D, HT, dyslipidemia, HF & COPD |
Tachycardia, chest pain, pulmonary edema, elevated troponin |
4 years |
Sinus rhythm, Ventricular and premature atrial complexes, Nonspecific ST-segment changes |
Conservative medical management, left upper extremity angiogram with stent placement (unsuccessful), planned interval operative intervention for the SA |
N/A |
N/A |
|
Mustapić et al., 2024 [3] |
1 |
62 |
M |
T2D & hypercholesterolemia |
Acute NSTEMI, instability, ST elevation in lateral and anteroseptal leads |
2 days |
Initial: Diffuse ST depression (inferior/lateral), ST elevation in aVR, Post-op: ST elevation in lateral and anteroseptal leads |
Coronary angiography and aortic arch angiography, PTA of the LSA using balloon dilatation and stent placement, PCI of the obtuse marginal artery with two drug-eluting stents
|
Resolution of ECG changes, Hemodynamic stabilization |
3 months
|
|
Real et al., 2021 [7] |
3 |
61 |
F |
Familial hypercholesterolemia, Carotid artery stenosis |
Unstable angina with ST depression |
3 years |
Extensive ST-segment depression, ST elevation in aVR and V1
|
Balloon-expandable endoprosthesis implantation |
Resolution of symptoms
|
5 months
|
|
72 |
M |
T2D, CKD |
NSTEMI with prolonged chest pain
|
5 months |
No ST/T changes |
Balloon-expandable stent |
Resolution of symptoms |
6 months |
||
|
76 |
M |
HT, carotid artery stenosis |
Asymptomatic, detected during follow-up due to an absent left arm pulse |
1 year |
Sinus rhythm, No ischemic changes |
Balloon-expandable stent, Predilatation with Mustang balloon |
Resolution of symptoms |
10 months |
||
|
F: Female, M: Male, IHD: Ischemic heart disease, CKD: Chronic kidney disease, COPD: Chronic obstructive pulmonary disease, HT: Hypertension, T2D: Type 2 diabetes, CAD: Coronary artery disease, N/A: Not applicable, PAD: Peripheral artery disease, CVA: Cerebrovascular accident, T1D: Type 1 diabetes, HF: Heart failure, STEMI: ST-Elevation Myocardial Infarction, NSTEMI: Non-ST-elevation Myocardial Infarction, ECG: Electrocardiogram, LCX: Left circumflex artery, LSA: Left Subclavian Artery, LIMA: left internal mammary artery, LCA: Left Coronary Artery, PCI: Percutaneous coronary intervention, ACE: Angiotensin-Converting Enzyme, SA: Subclavian artery, PTA: Percutaneous transluminal angioplasty, LVEF: Left ventricular ejection fraction, LAD: Left anterior descending |
||||||||||
The diagnosis of CSSS is often challenging and relies on clinical suspicion, particularly when certain physical signs are present, such as an inter-arm blood pressure difference of ≥15 mmHg [7]. This patient exhibited a striking 50/25 mmHg difference between the right and left arms, strongly suggesting subclavian artery involvement. Shadman et al. reported that a 15-mmHg difference could indicate clinically significant SAS [12]. However, the primary limitation of blood pressure measurement is that it provides only an indirect assessment [1]. While digital subtraction angiography remains the traditional gold standard for imaging SAS, it has increasingly been replaced by duplex ultrasound, CT angiography, or magnetic resonance angiography [9]. For the current patient, the electrocardiogram was unremarkable, and echocardiography revealed no significant abnormalities. CT angiography of the coronary grafts and thoracic outlet demonstrated complete occlusion of the left SA just proximal to the LIMA ostium.
Axillary-to-axillary or carotico-subclavian bypass are major surgical techniques for the treatment of SAS [1]. However, percutaneous transluminal angioplasty with or without stenting and subclavian artery bypass surgery are the most commonly employed methods for managing SSS. Among these, percutaneous transluminal angioplasty is generally preferred due to its lower morbidity and shorter hospital stay [1]. The choice of endovascular intervention in this case as first-line therapy aligns with current evidence-based recommendations, as Jahic et al. established percutaneous intervention as the preferred initial approach for subclavian artery stenosis, with success rates ranging from 84-100% for stenotic lesions [13]. However, this case presented the additional challenge of complete occlusion, which typically carries lower success rates [13]. Utilizing both radial and femoral access for optimal visualization improved the procedural outcome. Steinberger et al. reported treating a similar case with dual access, which resulted in complete resolution of symptoms [14]. A balloon-expandable peripheral stent was used in the current patient, as in five of the reviewed cases, because it is preferred for lesions requiring precise positioning and offers favorable outcomes [1,3,7,10].
Management of CSS also involves secondary prevention following CABG, which includes controlling risk factors and using therapies such as antithrombotic agents and statins [4]. Antiplatelets, lipid-lowering agents, renin–angiotensin–aldosterone system inhibitors, and beta-blockers are among the therapies utilized in CSSS cases [1,4,10].
Conclusion
Coronary subclavian steal syndrome can present shortly after CABG. Percutaneous endovascular stenting via a dual approach might offer good long-term outcomes.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Written informed consent was obtained from the patient for publication.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: FJA, JG, and HHM were significant contributors to the conception of the study and the literature search for related studies. SHT was the radiologist who performed the assessment of the case. SSA and MMA were involved in the literature review, study design, and manuscript writing. KFH and SHK were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. SSA and MMA confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: ChatGPT version 5.2 (OpenAI) was used solely for language editing, paraphrasing, and improvement of clarity and grammar in this manuscript. The artificial intelligence tool did not contribute to the study design, data collection, data analysis, data interpretation, or the generation of scientific content. All outputs produced with the assistance of ChatGPT were carefully reviewed, verified, and approved by the authors. The authors take full responsibility for the accuracy, integrity, and originality of the entire manuscript.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Unusual Metastasis from Follicular Thyroid Carcinoma: A Case Report and Literature Review
Abdulwahid M. Salih, Deari A. Ismaeil, Rawa M. Ali, Ari M. Abdullah, Karzan M. Salih, Shko H....
Abstract
Introduction
Follicular thyroid carcinoma (FTC) is a type of well-differentiated thyroid carcinoma. It has a poorer prognosis, is more metastatic, and has characteristics different from papillary thyroid carcinoma. It tends to metastasize hematogenously, usually to the bones and lungs. This study aims to present a rare case of FTC metastasis to the peritoneum and thigh of a patient with a literature review.
Case presentation
A 70-year-old patient presented with a right thigh mass. Magnetic resonance imaging of the right thigh revealed an intramedullary lesion in the femoral diaphysis. The thyroid gland was firm, with no evidence of enlargement. A computed tomography scan showed several peritoneal nodules. A core biopsy of the right upper femoral lesion and peritoneal mass was performed. Histopathologic findings and immunohistochemical analysis confirmed metastatic FTC with thyroid origin.
Literature review
Unlike widely invasive FTC, minimally invasive FTC has a better prognosis and is less metastatic. Imaging, histopathologic examination, and immunohistochemistry can help in arriving at a diagnosis of FTC. Mutations of PPAR-γ and RAS are associated with FTC. Radioiodine treatment and suppressive therapy for the thyroid-stimulating hormone have been shown to improve survival rates of FTC, with postoperative follow-ups and treatment being important.
Conclusion
Metastasis of FTC to the peritoneum is rare and could lead to a late diagnosis. Proper diagnosis with confirmatory tools such as immunohistochemistry and adequate treatment are critical.
Introduction
Follicular thyroid carcinoma (FTC) is an infrequent type of thyroid carcinoma that represents approximately 10% of all thyroid cancers [1]. Together with papillary thyroid carcinoma (PTC), they are classified as well-differentiated thyroid malignancies, which make up over 85% of all thyroid carcinoma cases. FTC, however, has genetic mutations, pathological characteristics, and a poorer prognosis different from PTC [2]. Follicular adenomas occur more frequently than follicular carcinomas. Follicular carcinomas are distinguished from follicular adenomas by microscopic evidence of vascular or capsular invasion [3]. Follicular thyroid cancer tends to spread through the bloodstream, which may account for its higher rate of distant metastasis compared to PTC [4]. Spreading via the lymphatic system can also occur but is rarer [3]. Distant metastasis occurs in around 6-20% of cases of FTC, with the bones and lungs being the most affected sites [4]. The tumor tends to present at a relatively older age and is associated with a higher rate of mortality compared to PTC [5]. The mortality rate of FTC is around 10–30% [6]. Metastasis is considered the most critical factor influencing survival outcomes in FTC. Sometimes, distant metastases could be the first sign of the disease, as some cases are only found after the cancer has spread to other body parts, while in others, they can appear following initial treatment [2]. Metastasis of FTC to the peritoneum is exceedingly rare. This study aims to showcase a very rare case of FTC metastasis to the peritoneum, along with the patient's thigh, combined with a literature review. The cited references in this case report have been assessed for reliability [7] and compiled with CaReL guidelines [8].
Case presentation
Patient information
A 70-year-old female patient presented with a right thigh mass that had been present for two weeks. She is a non-smoker with a known history of diabetes mellitus, currently managed with anti-diabetic medication. The patient underwent thyroid surgery in 2011. However, the histopathology report from the procedure was unavailable. She is presently taking levothyroxine 100 mcg once daily.
Clinical findings
The patient was vitally stable. On examination of the neck, the thyroid gland was found to be firm bilaterally upon examination, with no evidence of enlargement (Figure 1). No palpable cervical lymphadenopathy was detected. A small lymph node identified during evaluation demonstrated only reactive changes on histological examination.
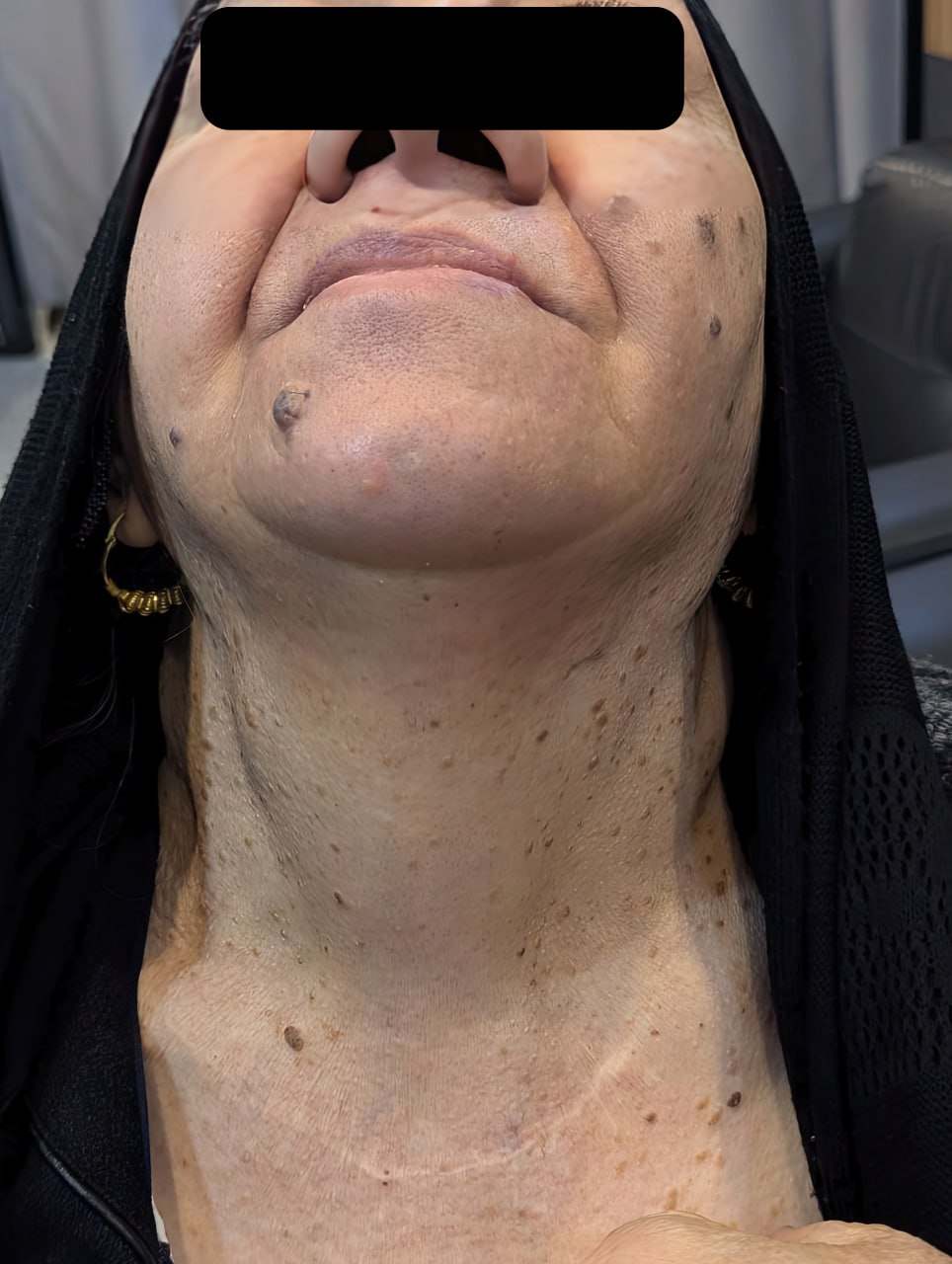
Diagnostic approach
Magnetic resonance imaging of the right thigh revealed a 35 × 48 mm intramedullary lesion in the femoral diaphysis. The lesion demonstrated low signal intensity on T1 and intermediate signal intensity on T2 with homogeneous post-contrast enhancement. It was associated with cortical destruction and extraosseous soft tissue extension. These imaging features are suggestive of metastatic involvement, warranting histopathological confirmation. A computed tomography scan of the chest and abdomen revealed multiple lung nodules, with the largest measuring 27 mm. Additionally, several peritoneal nodules were identified in the right and mid-anterior abdominal regions, the largest measuring 18 mm. These findings were suggestive of metastatic disease.
A core biopsy of the right upper femoral lesion was performed, confirming metastatic carcinoma. Immunohistochemical analysis suggested a thyroid origin. Laboratory investigations included thyroid function tests, which revealed that thyroid-stimulating hormone (TSH) level was 0.548 µIU/mL (normal range: 0.8–6.0 µIU/mL), free T4 was 21.5 pmol/L (normal range: 12.8–27 pmol/L), calcitonin (CATN) was <0.5 pg/mL (normal range: up to 9.82 pg/mL), thyroglobulin (Tg) was >500 ng/mL (normal range: 3.5–77 ng/mL), serum calcium was 9.83 mg/dL (normal range: 8.8–10.8 mg/dL), and parathyroid hormone (PTH) was 33.3 pg/mL (normal range: 15–65 pg/mL).
A neck ultrasound showed a remnant right lobe measuring 33 × 14 × 7 mm with a solid, heterogeneous echotexture. A smaller nodule measuring 12 × 7 × 4 mm of remnant tissue was detected below the lower pole of the previously noted remnant. In the midline of the neck, over the thyroid cartilage, an isoechoic, hypovascular remnant tissue measuring 15 × 6 × 4 mm was observed. The remnant left lobe measured 34 × 12 × 12 mm with a solid, heterogeneous echotexture containing a small TI-RADS 4 nodule measuring 6 × 6 × 4 mm. The isthmus was absent.
Additionally, a few small, solid, hypoechoic, hypovascular nodules were observed in the operative bed, with the largest measuring 5 × 3 mm in the left thyroid bed. Small lymph node-like structures were also noted. The patient underwent a core biopsy of the peritoneal mass. Histopathologic examination showed cores of fibrofatty tissue infiltrated by sheets of variably sized follicles that were lined by cuboidal cells with a moderate amount of eosinophilic to clear cytoplasm and round, hyperchromatic nuclei, some of which had conspicuous nucleoli. Eosinophilic colloid material was present in the lumina of some of the follicles. There was no notable mitotic activity or necrosis. There was a mild lymphoplasmacytic infiltrate in the fibrous tissue (Figure 2). Immunohistochemistry showed positive staining of the tumour cells for CK7 (cytoplasmic staining of moderate intensity in 70% of the tumour cells), PAX-8 (nuclear staining of moderate intensity in 100% of the tumour cells), and TTF-1 (nuclear staining of strong intensity in 100% of the tumour cells). These findings confirmed the diagnosis of metastatic follicular thyroid carcinoma to the peritoneum (Figure 3).
Therapeutic intervention
Following a comprehensive evaluation by a multidisciplinary team, the patient was scheduled for radioactive iodine ablation and subsequently underwent a total revision thyroidectomy. Both recurrent laryngeal nerves were successfully preserved. The excised tissue was submitted for histopathological examination, which revealed nodular Hashimoto’s thyroiditis with adenomatoid nodules and multiple reactive lymph nodes but no malignancy. The patient’s vital signs remained stable with smooth postoperative recovery. The peritoneal metastasis was managed with a plan for radioactive iodine ablation. Considering the patient’s clinical stability and the iodine-avid characteristics of the lesions, she was deemed an appropriate candidate for the therapy and was subsequently scheduled to receive it.
Follow-up and outcome
The patient recovered well postoperatively without complications. She was subsequently referred for radioactive iodine ablation and to an oncologist for ongoing follow-up and management. Her condition remains clinically stable, and she continues to be monitored pending treatment.
Discussion
Well-differentiated thyroid cancers like FTC and PTC will rarely metastasize to locations other than bones, lungs, and lymph nodes. Nonetheless, the occurrence of this small probability can lead to a late diagnosis [9]. Distant metastases to sites such as the brain, liver, breasts, muscles, kidneys, and skin are uncommon or occur infrequently. Distant metastasis is the most critical negative prognostic indicator; around 50% of affected patients survive beyond 10 years [10]. There is scant documentation of FTC metastasis to the peritoneum or abdominal area, with only a few case reports [11]. A struma ovarii containing FTC and metastasizing or spreading to the peritoneum can also occur [12, 13]. Mesentery metastasis of said carcinoma is rare as well [14]. Some cases of differentiated thyroid carcinoma metastases or spread to the peritoneum in literature have been reviewed in (Table 1) [11-15]. Iodine-sufficient and iodine-deficient regions show different ratios of papillary to follicular thyroid carcinoma, with iodine-deficient areas typically having lower ratios. Consequently, dietary iodine supplementation causes an increase in the said ratio [16].
|
Author and year of publication |
Age |
Sex |
Diagnosis |
Clinical findings |
Imaging |
Immunohistochemical tests |
Treatment |
Postoperative care |
Outcome |
|
Hamdy et al. 2024 [12] |
22 |
F |
left ovarian highly differentiated FTC from struma ovarii with peritoneal and omental spread. |
Iliac fossa pain, hypomenorrhea, & palpable lower abdominal mass. |
US: multicystic pelviabdominal lesion. Post-contrast MRI: midline multi-locular pelviabdominal left ovarian cystic mass. |
IHC: +ve TTF1, & Tg. |
Left salpingo-oophorectomy, omental & peritoneal biopsies, & thyroidectomy. |
Radioactive iodine treatment, & levothyroxine. |
Is in stable condition. |
|
Porntharukchareon et al. 2023 [11] |
44 |
F |
FTC metastasis to peritoneum. |
Multiple peritoneal nodules, low FT4 & normal TSH levels. |
CT of the peritoneum negative. US: small left thyroid nodule. |
IHC: +ve CK-7, Tg, & TTF-1. |
Total thyroidectomy. |
Radioactive iodine treatment. |
Is in stable condition. |
|
Chwa et al. 2023 [14] |
62 |
M |
Metastatic FTC on mesentery. |
Mesentery mass during check-up. |
PET/CT: mesentery mass. Chest CT: enhancing nodule in the left lower lobe. |
IHC: < 3% Ki-67. |
Lung lower lobe wedge resection, mesentery mass, & small bowel segment removed. |
Radioactive iodine treatment. |
Is in stable condition. |
|
Miles et al. 2016 [15] |
68 |
F |
Primary pelvic PTC metastasis to peritoneal surface. |
Urinary retention. |
US: heterogeneous pelvic mass. MRI: deep central pelvic mass like a uterus solid leiomyoma with extensive central necrosis. |
IHC supported the diagnosis of thyroid carcinoma. |
Surgically resected retroperitoneal mass, total hysterectomy, resected superficial peritoneum tumor implants, & elective total thyroidectomy after 3 months. |
Radioactive iodine treatment. |
Is in stable condition. |
|
Choi et al. 2012 [13] |
33 |
F |
Peritoneal dissemination of follicular carcinoma arising from struma ovarii. |
Enlarged uterus. |
MRI: bilateral par-ovarian solid tumors & unusual metastatic lesions manifestation in the pelvic cavity. |
Peritoneal mass HPE: thyroid gland enveloped in a fibrous capsule. |
Exploratory laparotomy under the impression of bilateral ovarian malignancy, total thyroidectomy. |
Radioactive iodine treatment. |
Is in stable condition. |
When it comes to FTC, it can be cytologically very similar to follicular thyroid adenoma, unlike papillary carcinoma, which is typically easy to diagnose due to its distinctive nuclear features and growth pattern. In such situations, the growth pattern of the tumor (particularly the presence of capsular and/or vascular invasion) can be used to differentiate carcinoma from adenoma [17]. Follicular thyroid adenomas and FTC also have distinct microarray expression profiles, which set them apart from each other, with FTC showing more overexpression of autotaxin, EMMPRIN, GADD153, and galectin-3 mRNAs, and follicular thyroid adenomas showing increased TFF3 mRNA expression [18]. Follicular thyroid cancer can be classified into minimally invasive FTC, widely invasive FTC, and encapsulated angioinvasive FTC, with minimally invasive FTC having a better prognosis and being less metastatic. In contrast, widely invasive FTC is typically associated with a larger tumor, occurring in older individuals, more invasive growth into the thyroid, and distant metastasis [2]. Both minimally invasive FTC and widely invasive FTC show a lack of cytological atypia. Histological features required for FTC diagnosis include angioinvasion and/or invasion that penetrates the full thickness of the tumor-surrounding capsule [17]. Multiple factors affect FTC prognosis, including the presence of residual tumor after surgery, the patient's age, whether there is local or distant metastasis, the size of the tumor, and the tumor's pathological classification [1]. On ultrasonography, the presence of microcalcifications, which may indicate the presence of psammoma bodies, is rare in FTC and common in PTC. At the same time, both PTC and FTC could have rim and coarse calcifications [16].
Immunohistochemistry can be handy in confirming the thyroid origin of a metastatic tumor [19]. In this case, each of CK7, PAX-8, and TTF-1 were utilized to diagnose and confirm metastatic origin. TTF-1 and PAX-8 can be produced in the lung and kidney, respectively. However, they are co-expressed with one another in thyroid follicular cells, highlighting the importance of this specific combination for establishing the thyroid of the tumor [20]. CK7 is a type of cytokeratin with a low molecular weight, an intermediate filament protein specific to epithelial cells, and it is typically found in glandular epithelium. It is more commonly used in diagnosing tumors outside the thyroid, where it helps determine the tumor’s origin, rather than in tumors within the thyroid gland itself. It may also be used with CK20 to help with the diagnosis, with CK7 positivity accompanied by CK20 negativity being found in a few organs including the thyroid [19]. The thyroid-specific enhancer-binding protein TTF-1 can also help confirm a tumor’s thyroid origin, but not in differentiating among different thyroid lesions, with positive results found in around three-quarters of thyroid carcinoma metastases [19]. Li et al. utilized TTF-1 and CK7 positivity, along with thyroglobulin and partial CD56 positivity, in their immunohistochemical analyses to help confirm metastatic FTC in a chest wall lymph node [21].
Thyroglobulin is close to TTF-1 when it comes to sensitivity in identifying primary and secondary thyroid follicular lesions, but with these antibodies together, their diagnostic sensitivity may increase [19]. Serum thyroglobulin can be a dependable marker for detecting metastasis or recurrence since it has a 30-hour half-life following a total thyroidectomy [14]. Olejarski et al. report a 73-year-old with a right thigh soft tissue mass, surrounding a right proximal-mid femoral prosthesis on a computed tomography scan. A core biopsy of the mass revealed a follicular lesion showing positive results for TTF-1 and thyroglobulin, indicating a metastatic tumor of thyroid origin. The patient showed an elevated serum thyroglobulin indicative of a significant volume disease [22]. Genetic mutations associated with FTC include mutations of PPAR-γ and the mitogen-activated protein kinase activator and PI3K-AKT pathways known as RAS, which is frequently found in follicular adenomas, indicating a role in its early tumorigenesis. Other mutations are required to transform the adenoma into a carcinoma, such as PIK3CA mutations and PTEN, a tumor suppressor gene [16].
When the cytological or ultrasound findings indicate a higher risk of cancer, or if malignancy is present and to allow for radioiodine treatment postoperatively, total thyroidectomy may be considered [23]. Grani et al. found several studies showcasing that patients with FTC who were given radioiodine treatment had better chances of survival than those patients who did not. Another one showed that patients with distant metastases had improved chances of survival with radioiodine treatment, and in those without distant metastases, it increased local control. They also found several studies that showed better results with suppressive therapy for the thyroid-stimulating hormone after the initial treatment. External beam radiotherapy is also linked to better relapse-free survival for FTC when treating local or distant areas [16]. Post-therapy 131I whole-body scintigraphy and 131I single photon emission computed tomography/computed tomography are essential for patient management in differentiated thyroid carcinomas due to their high sensitivity and specificity [10]. Miles et al., for example, made use of whole-body radioactive iodine scanning for their patient with primary pelvic PTC metastasis to the peritoneal surface after the surgeries to follow up on the patient’s condition. In it, two faint areas of activity were seen on the right side of the thyroid region, likely indicating small remnants of thyroid tissue, along with a small but clearly defined area of activity in the pelvic region, helping in the patient’s management as they got better [15].
Conclusion
The rarity of FTC metastasis to locations like the peritoneum could lead to a late diagnosis and complications. Thus, it is critical to consider this probability, make proper diagnoses with tools such as immunohistochemistry, and treat accordingly.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Written informed consent was obtained from the patient for publication.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: AMS and DAI were significant contributors to the conception of the study and the literature search for related studies. AHA was the radiologist who performed the assessment of the case. RMS was the pathologist who performed the histopathological diagnosis. TOS and HAA were involved in the literature review, study design, and manuscript writing. KMS, AMA, and SHH were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. SSA and MMA confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: ChatGPT version 5.2 (OpenAI) was used solely for language editing, paraphrasing, and improvement of clarity and grammar in this manuscript. The artificial intelligence tool did not contribute to the study design, data collection, data analysis, data interpretation, or the generation of scientific content. All outputs produced with the assistance of ChatGPT were carefully reviewed, verified, and approved by the authors. The authors take full responsibility for the accuracy, integrity, and originality of the entire manuscript.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Atypical Sigmoid Volvulus in an Adolescent: A Case Report and Literature Review
Dana T. Gharib, Dara Ahmed Mohammed, Araz Aziz Abdulla, Othman O. Qadr, Karokh F. Hama Hussein,...
Abstract
Introduction
Sigmoid volvulus is an underrecognized diagnosis with potentially fatal outcomes in adolescents; the current study aims to present an adolescent with mild symptoms but was found to have extensive ischemic sigmoid volvulus.
Case presentation
An 18-year-old male complained of three days of moderate colicky abdominal pain, with decreased appetite and diarrhea. Physical examination revealed diffuse abdominal tenderness and exaggerated bowel sounds without abdominal distension. A contrast-enhanced computed tomography scan demonstrated evidence of twisting the sigmoid and a part of the left colon around each other but without signs of ischemia and perforation. Sigmoidoscopy showed volvulus with dusky ischemic mucosa. An immediate surgical intervention through Hartmann's procedure was performed. After three months of the operation, colostomy closure and colorectal re-anastomosis were performed.
Literature review
In a review of the literature, adolescents presented with symptoms of abdominal pain, swelling, vomiting, dehydration, and even shock in more severe cases. Despite the lack of data regarding the optimal long-term approach for sigmoid volvulus in adolescents, in a review of 63 cases, 77% of them received operative treatment, with sigmoidectomy being the predominant procedure. The total mortality rate was 6%, with an operative mortality rate of 8.1%.
Conclusion
Strangulated sigmoid volvulus should be considered in adolescents with abdominal pain despite having diarrhea and no clinically evident abdominal distention. Hartman’s procedure may result in a good outcome.
Introduction
During embryogenesis, the sigmoid colon arises from the primitive gut tube, more specifically, the posterior portion of it, which is referred to as the hindgut. The upper anal canal, rectum, sigmoid colon, descending colon, and the distal third of the transverse colon are all structures derived from the hindgut. As embryonic development progresses, the hindgut elongates and undergoes a sequence of intricate rotations and fixations, ultimately positioning the sigmoid colon in the lower left quadrant of the abdomen. The sigmoid colon receives its blood supply from the inferior mesenteric artery [1]. Like the transverse colon, the sigmoid colon has a mesocolon that can be subject to twisting around itself and cause a closed-loop obstruction, this is referred to as sigmoid volvulus, which was first documented by von Rokitansky in 1836. In Western countries, sigmoid volvulus represents less than 5% of all colonic obstructions, reaching up to half of the obstruction cases in the Eastern part of the world. More specifically, the Middle East, Asia, South America, Africa, and Northern and Eastern European countries are considered endemic regions. This condition has a peak incidence between the 40s and 80s of someone's age, with males being predominantly affected with ratios varying from 2:1 to 10:1 [2, 3].
The anatomical structure of the sigmoid colon is a critical factor in predisposing individuals to sigmoid volvulus. These include a constricted base of the sigmoid mesentery, excessive length of the sigmoid colon, and dolichomesentery, which is defined as a mesentery that is broader than it is in length. The latter factor, which is more common in males and their narrower pelvic inlet, explains the higher rate of sigmoid volvulus in this gender, as it also hinders spontaneous detorsion. Other risk factors like colitis, prune belly syndrome, Hirschprung’s disease, pregnancy, laxative abuse, and chronic constipation have also been described. Patients commonly present with complaints of abdominal pain, nausea, vomiting, and abdominal swelling. However, in adolescents, the diagnosis can be overlooked or delayed due to this condition's infrequency in this age group. As a result, morbidity and mortality related to this can be high due to closed-loop obstruction, bowel ischemia, and hypovolemic shock [3, 4].
The current study aims to report an adolescent who presented with only moderate colicky abdominal pain, decreased appetite, and fever but was later found to have ischemic sigmoid volvulus. This case report was written in line with CaReL guidelines, and the credibility of the references was checked according to the well-known predatory list journals [5, 6].
Case presentation
Patient information
An 18-year-old male with a body mass index of 35.49 kg/m2 presented to the emergency department with three days of moderate colicky abdominal pain. There were no known aggravating or relieving factors, but he reported a decreased appetite and sometimes had diarrhea. Although he did not experience vomiting, he had a persistent fever for the past two days before the presentation. He was a non-smoker and non-alcoholic with unremarkable past medical or surgical history.
Clinical findings
His vital signs showed a high body temperature (38 c0), normal blood pressure (110/80 mmHg), and tachycardia (114 bpm). Physical examination revealed diffuse abdominal tenderness and exaggerated bowel sounds without abdominal distension. There were no pallor, jaundice, cervical lymphadenopathy, and edema. A digital rectal examination revealed an empty rectum with no mass or perianal lesion.
Diagnostic approach
White blood cells (28 x 109 /L) and C-reactive protein (110 mg/ dl) were abnormal. The renal function tests were normal. Transabdominal ultrasound (U/S) showed no significant finding. A contrast-enhanced computed tomography (CT) scan of the abdomen demonstrated a large bowel distention (9 cm in maximum axial diameter) with evidence of twisting of the sigmoid and a part of the left colon around each other along their mesentery causing total distal bowel obstruction without sign of ischemia and perforation (Figure 1).
Therapeutic intervention
There was obstipation on the day before the surgical intervention. Conservative treatment started with intravenous fluid and antibiotics, and then an urgent sigmoidoscopy was performed. It showed apparent volvulus with dusky ischemic mucosa around it (Figure 2). An immediate referral to surgery by a gastroenterologist was made. Through midline incision, the intraoperative findings confirmed sigmoid volvulus which was resected, and then Hartmann's procedure was performed (Figure 3).
Follow-up
After three months of the operation, colostomy closure and colorectal re-anastomosis were performed. The patient was doing well and had normal physical status and vital signs.
Discussion
Intestinal volvulus is rarely encountered in the adolescent age group, with a few cases reported in recent years summarized in (Table 1) [2,4,7-13]. This condition requires the presence of a redundant, long, and mobile section of the intestine, coupled with a relatively narrow mesenteric attachment. This configuration leads to the fixation points at both ends of the intestinal segment being positioned close to each other, which facilitates the twisting or rotation of the segment. The sigmoid colon offers the perfect conditions for this twisting to happen. It can exhibit significant redundancy and mobility, with the fixation points at the lower part of the sigmoid and the junction where the sigmoid transitions into the rectum frequently situated close to one another. This elongated, redundant sigmoid colon with a tapered mesentery is called dolichosigmoid [14].
|
Author (year)reference |
No. Case |
Age (year) |
Sex |
PSH |
Presentation |
PHE/ CF |
Comorbidity |
Imaging findings |
Gangrene/ ischemia |
Management |
Follow-up (months) |
Recurrence |
|
Visalli et al. (2021)7 |
1 |
13 |
F |
No |
Constipation, Abd. Pain, LOA |
greatly distended, non-tender, and painless abdomen, without muscle guarding or rebounding tenderness |
Chronic Constipation |
SV |
No |
a water-soluble fluoroscopy guided contrast enema |
2 |
No |
|
Choi et al. (2020)8 |
1 |
16 |
M |
No |
vomiting, diarrhea, distension, Abd. pain |
abdominal distension with mild abdominal tenderness but without rebound tenderness, and a subtle metallic bowel sound. |
- |
SV |
No |
Rectal tube but recurrence occurred two times. definitive management by laparoscopic sigmoid colectomy |
9 |
Yes |
|
Esmat et al. (2020)9 |
1 |
19 |
M |
No |
Abd. pain, constipation, vomiting |
hypoactive bowel sounds, diffuse abdominal tenderness, and severe distention without guarding and rebound tenderness. |
- |
SV |
No |
a trans-rectal endoscopic detorsion, later anterior sigmoid resection and primary rectosigmoid anastomosis |
<1 |
Yes |
|
Godosis et al. (2020)10 |
1 |
10 |
M |
No |
Abd. pain, vomiting |
child appeared as a heavily sick patient, sweaty with weakness. His abdomen was flatulent in the two upper quadrants and totally flat and stiff in the two lower ones. Bowel sounds were absent |
- |
SV |
No |
A sigmoidectomy with a primary end to-end anastomosis of the descending colon with the upper rectum plus appendectomy were performed |
6 |
No |
|
Bhandari et al. (2019)4 |
1 |
14 |
M |
No |
Abd. Pain and distention, vomiting |
Signs of dehydration, gross Abd. distention, hyper-resonance with mild tenderness. Sluggish bowel sounds |
Constipation, developmental 3 delay, mental retardation |
SV |
No |
Lapartotomy, redundant sigmoid colon was resected after detortion and resection anastomosis was performed. |
<1 |
No |
|
Hassan et al. (2018)11 |
1 |
11 |
F |
No |
Abd. Pain and distention, vomiting, , and constipation |
Dehydrated, severely distended abdomen with diffuse tenderness, guarding, and tympanic to percussion with no shifting dullness. Sluggish bowel sounds. |
- |
SV |
Yes |
Hartmann's procedure was performed with a proximal end colostomy. |
<1 |
No |
|
Chang et al. (2017)12 |
1 |
12 |
M |
No |
Abd distention, nausea, poor appetite |
Massively distended abdomen. |
Autism |
SV |
No |
laparotomy with mesosigmoidoplasty for detorsion of the sigmoid |
3 |
No |
|
Emeka et al. (2022)2 |
1 |
14 |
M |
No |
Abd. Pain, vomiting |
Abd. distention, with marked tenderness in the suprapubic and both iliac fossae. Hyperactive bowel sounds. |
- |
SV |
No |
Sigmoidectomy with colorectal anastamosis |
<1 |
No |
|
Patel et al. (2014)13 |
1 |
14 |
F |
No |
abdominal distention, lower abdominal pain and absolute constipation |
abdomen was soft and diffusely distended and tympanic |
acquired microcephaly, central motor disorder severe learning disability, repetitive, chronic constipation and iron deficiency anaemia |
SV |
No |
emergency colonoscopic reduction and decompression followed by elective sigmoid colectomy |
6 |
No |
|
PHE/CF; Physical examination/clinical findings, Abd: abdominal, LOA: loss of appetite, PSH: past surgical history, SV: sigmoid volvulus |
||||||||||||
There is controversy regarding the development of dolichosigmoid. Some authors suggest that a congenitally long sigmoid colon, which lengthens over the years if a patient has chronic constipation, is the culprit of sigmoid volvulus. Proponents of this theory highlight that sigmoid volvulus tends to run in families, shows a higher prevalence in males, and exhibits a significant racial predisposition. They also note that certain tribes, such as the Pathans in Pakistan and Akambas in Kenya, show higher rates of this condition than their neighboring populations. Furthermore, the higher incidence sigmoid volvulus in Indians, who are studied to have a high incidence of dolichosigmoid in contrast to other ethnic groups, strengthens this side. Nevertheless, this congenital theory does not account for the rarity of the disorder in infants and children. Although dolichomegacolon can lead to chronic constipation and vice versa, the causal connection between the two remains unconfirmed. However, sigmoid volvulus in adolescents could favor the dolichomegacolon as the cause of chronic constipation. It is not likely for chronic constipation to cause such anatomical change from a young age, and it usually requires years before it manifests in adulthood. More studies specifically investigating these theories are still needed to establish this. [12-15]
Moreover, the differences in the frequency of sigmoid volvulus across various regions are thought to be associated with the consumption of a diet rich in fiber, particularly prevalent in Eastern regions. This high-fiber diet elongates both the sigmoid colon and its mesentery, creating a predisposed anatomical environment that makes it more susceptible to volvulize. In Western nations, where diets are characterized by low fiber content and high fat intake, colorectal cancer and diverticular disease emerge as the principal causes of colonic obstruction. For instance, in two of the cases in our review in the reported case by Visali et al. and Patel et al., who were 13 and 14 years old, respectively, they had a history of chronic constipation in line with that hypothesis; however, the current patient and the other cases in Table 1 did not complain or mention chronic constipation, which is suggestive of another pathophysiologic mechanism at play for patients in this age group [7,13,14].
The sigmoid colon can withstanding higher levels of intraluminal pressure compared to other segments of the large intestine, allowing the bowel wall to stay functional for several days. Nevertheless, heightened peristaltic activity in the proximal colon and increased fluid secretion lead to distension and elevated pressure within the volvulated segment. Bacterial fermentation further contributes to gas accumulation within the obstructed loop, exacerbating the swelling and raising the intraluminal pressure. If not salvaged in time, this increased pressure leads to vascular compromise and eventual ischemia and necrosis. This explains the severe distention reported by Esmat et al., 19-year-old male, who presented with abdominal pain, constipation, and vomiting for four days, which is enough time for colonic fluid secretion and bacterial fermentation in the volvulus to cause the severe distention of the patient complained of. However, what sets the current patient apart is the lack of abdominal distention and only moderate pain despite the onset of the symptoms for three days. What is more striking is that the patient was found to have dusky ischemia on sigmoidoscopy requiring urgent operative management. Furthermore, Hassan et al. described a case involving an 11-year-old patient who was discovered to have a severely swollen, dark, gangrenous sigmoid with a narrow mesentery and thrombosis of the vessels. This case underscores the severity and seriousness of the condition, particularly in adolescents, where it is less commonly included in the differential diagnosis [9, 11, 14].
It is essential to highlight that for the twist to result in a clinically relevant obstruction, it must exceed 180°. Twists less than 180° are considered physiological volvulus and typically do not have a significant clinical impact. In contrast, a torsion that causes lumen obstruction and exceeds 180° is classified as obstructive volvulus. When the torsion surpasses 360°, it is called strangulating volvulus. There is frequently a notable delay, averaging between 3 to 4 days, from the onset of sigmoid volvulus to its evaluation. Due to the often severe and rapid onset of symptoms associated with endemic sigmoid volvulus, patients typically present with signs indicative of an acute abdomen. These symptoms commonly include abdominal tenderness, severe pain, early onset of vomiting, bloody stools, and, in severe cases, hemodynamic instability. In chronic or indolent sigmoid volvulus, there may be partial obstruction of the sigmoid colon that allows for the passage of liquid stools, leading to the paradoxical occurrence of diarrhea. In the present case, although the patient reported diarrhea, suggestive of potential partial obstruction, the diagnostic evaluation revealed strangulation with impaired blood flow. Additionally, it is crucial to recognize that in pediatric cases, sigmoid volvulus often presents as repeated abdominal pain and hematochezia, which could be erroneously diagnosed as ischemic colitis or irritable bowel syndrome [7,12-15].
In 60 to 75 percent of sigmoid volvulus cases, a simple plain abdominal radiograph is often needed to confirm the diagnosis. These radiographs usually reveal dramatic colonic distension, sometimes accompanied by slight bowel enlargement. When the radiographs are inconclusive, a contrast enema can provide crucial diagnostic insights, proving particularly effective in children compared to adults. For instance, in the case of Visali et al., the contrast enema not only diagnosed but also resolved the volvulus without surgical intervention. Remarkably, the patient experienced no recurrence of symptoms throughout a two-month follow-up period. Computed tomography scans and magnetic resonance imaging offer nearly 100 percent diagnostic accuracy for sigmoid volvulus. Despite this high accuracy, their application is constrained by limited availability in endemic regions. Both imaging modalities effectively reveal the twisted pedicle of sigmoid volvulus as a characteristic whorled soft tissue mass. Endoscopy serves both diagnostic and therapeutic roles in managing sigmoid volvulus. The occurrence of mucosal spirals and blockage within the lumen at the rectosigmoid junction observed during endoscopy is considered pathognomonic for sigmoid volvulus. However, up to 15% of SV cases are typically identified only during laparotomy or post-mortem examination. Entities that could present similarly to sigmoid volvulus include neoplasms of the colon, colonic megacolon resulting from irregular motility, toxic megacolon, and ileosigmoid knotting [5-8, 14].
Management approaches for sigmoid volvulus focus on relieving the obstruction and preventing recurrence. The standard elective surgical approach is sigmoid resection with primary anastomosis. This procedure is performed through open laparotomy or laparoscopic methods, provided the large intestine can be adequately decompressed. Individuals who are effectively decompressed via endoscopy before ultimate resection have a recurrence rate approaching 0% [14].
Emergency resection and primary anastomosis are recommended if decompression is deemed unfeasible or when clinical signs indicate peritonitis or colonic ischemia. Intestinal ischemia signs include gangrenous mucosa observed while attempting decompression, bloody stool, increased body temperature and white blood cells, abdominal tenderness, and hemodynamic instability. In these scenarios, immediate patient resuscitation is essential, followed by exploration through a midline laparotomy. If the bowel is viable during laparotomy, the volvulus should be manually reduced and the twisted sigmoid colon resected. However, if a gangrenous bowel is found, detorsion should be avoided. Performing detorsion in the presence of gangrene risks releasing accumulated toxins and bacteria into the bloodstream, potentially leading to sepsis and cardiovascular collapse. [13-15]
In a review of 63 cases by Salas et al. involving children and adolescents with sigmoid volvulus, 49 patients received operative treatment, with sigmoidectomy (with or without primary anastomosis) being the predominant procedure. The total mortality rate was 6%, with an operative mortality rate of 8.1% and a neonatal mortality rate of 14%. [15].
Conclusion
Strangulated Sigmoid volvulus should be considered in adolescents with abdominal pain despite having diarrhea and no clinically evident abdominal distention. Hartman’s procedure may result in a good outcome.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Written informed consent was obtained from the patient for publication.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: DTG and DAM were significant contributors to the conception of the study and the literature search for related studies. OOQ was the radiologist who performed the assessment of the case. DH was involved in the literature review, study design, and manuscript writing. AAA, KFH, DAI, HHK, and HOA were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. DH and DTG confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: ChatGPT version 5.2 (OpenAI) was used solely for language editing, paraphrasing, and improvement of clarity and grammar in this manuscript. The artificial intelligence tool did not contribute to the study design, data collection, data analysis, data interpretation, or the generation of scientific content. All outputs produced with the assistance of ChatGPT were carefully reviewed, verified, and approved by the authors. The authors take full responsibility for the accuracy, integrity, and originality of the entire manuscript.
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Retraction

Retraction Note: Arteriovenous Fistula Creation for Hemodialysis in Patients with End-Stage Renal Disease with and Without Surgical Drain: A Randomized Control Trial
Lokish S. Jaswel, Narayan Oste, Satish Vaidy
The Original Article was published on February 10, 2025
This article has been retracted at the request of the publisher (Barw Publisher) following an internal investigation. After publication, the journal was informed that the manuscript had been submitted without the final approval of all listed authors, which constitutes a breach of the journal’s authorship and publication ethics policies. Multiple attempts were made to contact the corresponding author to clarify this issue; however, no response was received. Following discussion between the publisher and the Editor-in-Chief, a decision was made to retract the article in order to maintain the integrity of the scientific record.
The Editor-in-Chief and the publisher regret any inconvenience caused to readers.
