Emerging Evidence of IgG4-Related Disease in Pericarditis: A Systematic Review
Abstract
Introduction
Immunoglobulin G4-related disease (IgG4-RD) is a recently identified immune-mediated condition that is debilitating and often overlooked. While IgG4-RD has been reported in several organs, this study reviews cases where IgG4-RD caused pericarditis.
Methods
A systematic search was conducted from inception until March 1, 2025. All age groups and both sexes with confirmed pericarditis were included, along with the following inclusion criteria: 1) Patients with pericardial biopsy showing IgG4/IgG ratio of >40%. 2) Patients with pericardial biopsy revealing IgG4/HPF of >10. 3) Patients who had confirmed IgG4-RD from other organ biopsies through IgG4 staining, or diagnostic imaging suggestive of IgG4-RD, or pericardial biopsy with classic IgG4-RD histopathologic patterns, with elevated serum IgG4 levels, provided no other diagnosis was more likely.
Results
A total of 50 patients were included, with a mean age of 64.86±15.79 years. There were 36 (72%) males. The most common presenting symptom was dyspnea in 27 (54%) patients. Different pericardial involvements were reported, including pericardial thickening 37 (74%), constrictive pericarditis 28 (56%), pericardial effusion 23 (46%), pericardial calcification 6 (12%), and pericardial nodule 5 (10%). In 28 (56%) patients, only the pericardium was affected. In addition to the pericardium, eight (16%) patients had one other organ affected, and 11 (22%) patients had two additional organs affected. Two (4.5%) cases ended in demise.
Conclusion
Although rare, IgG4-RD can cause pericarditis, leading to pericardial thickening, effusion, constrictive pericarditis, or the formation of pericardial nodules. Treatment with corticosteroids or pericardiectomy has been associated with favorable outcomes.
Introduction
Immunoglobulin G4-related disease (IgG4-RD) is an immune-mediated, fibroinflammatory condition that affects multiple organs in the body. It is a slowly progressing and often debilitating disease, with the potential to be fatal in some cases [1]. The condition was first suspected in the pancreas where a subset of autoimmune pancreatitis was associated with elevated levels of serum IgG4, now termed autoimmune pancreatitis type I [2]. The discovery that individuals with autoimmune pancreatitis also develop extra-pancreatic fibroinflammatory lesions containing abundant IgG4-bearing cells, in addition to histopathological features consistent with that in the pancreas, which include; dense lymphoplasmacytic infiltration, storiform fibrosis, and either obliterative or non-obliterative phlebitis, contributed to the establishment of the concept of IgG4-RD as a unique clinical entity in 2003 [1-3]. For decades, conditions such as Riedel thyroiditis, sclerosing cholangitis, Mikulicz’s syndrome, hypertrophic pachymeningitis, and retroperitoneal fibrosis were considered distinct entities. However, they are now classified within the spectrum of IgG4-RD, as they have been found to share similar histologic features and present concurrently in some patients [4].
The worldwide prevalence and incidence of IgG4-RD are mostly underreported. Still, studies from Japan have revealed that the incidence of autoimmune pancreatitis increased from 0.8 to 3.1 cases per 100,000 people between 2007 and 2016, suggesting a swift rise in recognition of IgG4-RD within just a decade [4]. There is a higher prevalence among males, with the average age at diagnosis typically ranging from the fifth to sixth decade of life. However, classic presentations have also been documented in pediatric patients [1]. Cigarette smoking is the only well-documented modifiable risk factor associated with the development of IgG4-RD [5]. A genome-wide association study revealed that the FC-γ receptor IIb and HLA-DRB1 regions were associated with an increased susceptibility to IgG4-RD, indicating a potential genetic predisposition to its pathogenesis [6].
The pathophysiology of IgG4-RD remains incompletely understood. However, several critical components have been identified, including the migration of activated B cells to the site of inflammation, where they facilitate the expansion and differentiation of T cells into CD4+ cytotoxic T lymphocytes (CTLs). These CD4+ CTLs subsequently induce apoptosis by releasing perforins and granzymes. In response, activated M2 macrophages clear the apoptotic cells while also contributing to the activation of fibroblasts. This activation is promoted through several mediators, including IFN-γ (interferon-gamma), TGF-β (transforming growth factor-beta), and IL-1 (interleukin-1) from CD4+ CTLs, along with PDGF (platelet-derived growth factor) from activated B cells, and various factors from macrophages. As fibroblasts become activated, they secrete extracellular matrix proteins, leading to tissue remodeling and fibrosis. Over time, the progressive expansion of the extracellular matrix and increased cell proliferation contribute to the development of tumor-like masses and the subsequent enlargement of affected organs, as observed in clinical settings [7].
While IgG4-RD has been reported in multiple organs, this study is the first to comprehensively review cases where it affects the pericardium, leading to pericarditis.
Methods
Study design
The present systematic review was conducted per the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Literature search
A thorough systematic search was conducted across Scopus, PubMed, Web of Science, and Google Scholar databases to retrieve studies published from inception until March 1, 2025. The search employed the following keywords: “IgG4 OR IgG4RD OR immunoglobulin AND pericarditis OR tamponade OR pericardial OR pericardium OR serositis OR serosal”
Eligibility criteria
The inclusion criteria were restricted to English-language publications involving only human subjects, specifically case-control studies, cohort studies, cross-sectional studies, or case reports. Additionally, due to the limited number of studies on this topic, conference papers containing adequate information were also included.
All age groups, both sexes, with confirmed pericarditis through a combination of clinical examination, ECG (electrocardiography), diagnostic imaging (including echocardiography, magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET)), laboratory investigations, or histopathological examination of the pericardial tissue or fluid were included. Given the limited number of reports on this entity and a lack of standardized diagnostic criteria for IgG4-RD pericarditis, such as an established IgG4/IgG ratio or IgG4/high power field (HPF) in pericardial specimens or its necessity in the first place when other clues are suggestive of this disease, this review adopted an inclusive approach. With patients of either of the following criteria being included: 1) Patients with pericardial biopsy showing an IgG4/IgG ratio of >40% or reported as “increased”. 2) Patients with pericardial biopsy revealing IgG4/HPF of >10 or reported as “increased”. 3) Patients who had confirmed IgG4-RD from other organ biopsies through IgG4 staining, or diagnostic imaging suggestive of IgG4-RD, or pericardial biopsy with classic IgG4-RD histopathologic patterns, with elevated serum IgG4 levels, provided no other diagnosis was more likely.
The exclusion criteria included studies with incomplete information about the patients or the method of diagnosis. Patients who were more likely to have pericarditis due to causes other than IgG4-RD. Studies from journals with inadequate peer review and questionable reliability were excluded [8].
Study selection
The screening process commenced with two independent researchers who systematically reviewed the titles and abstracts of all identified studies. Following this initial assessment, a comprehensive full-text evaluation was conducted based on predefined inclusion and exclusion criteria. Studies that satisfied these eligibility criteria were subsequently selected for inclusion. In instances where disagreements emerged between the two researchers, a third author was consulted to mediate and resolve conflicts through discussion and consensus.
Data items
Data extraction was conducted using Microsoft Office Excel 2016. The following variables were collected for each study: first author’s name, year of publication, study design, country of origin, sample size, patient sex, age, past medical and surgical history, family history, presenting complaint, and duration of symptoms. Additionally, data regarding pericardial involvement were extracted, including the presence of pericardial thickening, constrictive physiology, pericardial calcification, and pericardial nodules. The presence or absence of pleural disease was also recorded.
If available, findings from diagnostic imaging modalities such as chest radiography, echocardiography, CT, MRI, PET, and right heart catheterization were documented. Laboratory results, when reported, were extracted for C-reactive protein (CRP), Erythrocyte sedimentation rate (ESR), N-terminal pro-brain natriuretic peptide (NT-proBNP), serum IgG4 levels, serum IgG4/IgG ratio, and any other notable laboratory parameters.
Furthermore, the number of organs affected by IgG4-RD, and the histopathological findings were collected. Treatment modalities, including both successful and unsuccessful interventions, were documented, along with follow-up duration and clinical outcomes.
Data analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS) version 26.0, which facilitated the quantitative synthesis of the information. Relevant variables were displayed in summary tables, with categorical data represented by frequency and percentage, and quantitative data summarized using the mean and standard deviation.
Results
Study selection
The literature search yielded 87 studies from the databases. During the initial screening, one study was removed due to duplication, four for being in a non-English language, and four were closed access/unretrievable. The 78 studies remained for screening through titles and abstracts. Of these, eleven were excluded from the title and three from the abstract due to irrelevancy.
During the full-text screening, two retrospective cohort studies were excluded even though they included patients with IgG4-RD causing pericarditis. As those studies also included patients with IgG4-RD affecting other organs without pericardial involvement. However, they did not provide specific details on the characteristics, diagnostic workup, or histopathological findings of IgG4-RD in the subset of patients with pericarditis. As a result, they did not meet the inclusion criteria for this systematic review. One study was excluded as there was insufficient information to diagnose IgG4-RD. Additionally, two other studies were excluded after the full-text screening because they did not report IgG4-RD as a cause of pericarditis. Letters to the editor and preprints were excluded, with six and one paper removed respectively. Two studies were excluded from journals with inadequate peer review. Ultimately a total of 50 studies were included in the current systematic review for analysis (Figure 1).
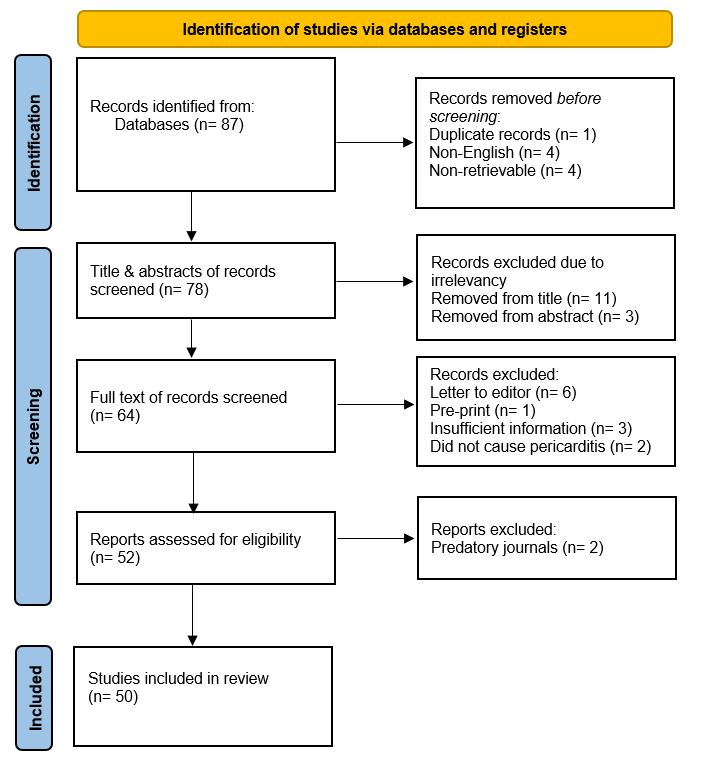
Characteristics of the studies
Of the included studies, 40 (80%) were case reports, and ten (20%) were conference abstracts. Japan 22 (44%) and the United States of America 17 (34%) had the most publications, followed by Korea 3 (6%), the other studies were all from different countries (Table 1, Table 2, Table 3) [9-58].
|
Author |
Year |
Country |
Study type |
No. cases |
Age (y) |
Sex |
Presenting complaint |
Duration |
PMH |
Pleural disease |
Pericardial manifestations |
Extra-pericardial IgG4-RD organ involvement |
|
Khodabandeh et al. [9] |
2010 |
USA |
P |
1 |
54 |
M |
Dyspnea |
N/A |
No |
Yes |
Thickening, constrictive pericarditis |
No |
|
Horie et al. [10] |
2012 |
USA |
P |
1 |
76 |
M |
Dyspnea, peripheral edema |
24 |
N/A |
Yes |
Thickening, constrictive pericarditis |
No |
|
Kabara et al. [11] |
2012 |
Japan |
C |
1 |
69 |
M |
Peripheral edema |
12 |
N/A |
Yes |
Pericardial effusion |
No |
|
Sekiguchi et al. [12] |
2012 |
USA |
C |
1 |
29 |
F |
Dyspnea, chest pain |
60 |
N/A |
Yes |
Thickening, constrictive pericarditis |
Pleural thickening |
|
Sekiguchi et al. [13] |
2012 |
USA |
C |
1 |
76 |
M |
Dyspnea, peripheral edema |
24 |
N/A |
Yes |
Thickening, constrictive pericarditis |
No |
|
Kassier et al. [14] |
2014 |
USA |
P |
1 |
75 |
M |
Peripheral edema |
N/A |
N/A |
No |
Thickening, constrictive pericarditis, effusion, calcification |
No |
|
Morita et al. [15] |
2014 |
Japan |
C |
1 |
60 |
F |
Referred for cardiac tamponade |
N/A |
N/A |
No |
Thickening, effusion, tamponade |
Lacrimal and parotid glands, mediastinal lymph nodes |
|
Seo et al. [16] |
2014 |
Korea |
C |
1 |
58 |
M |
Dyspnea, fatigue |
0.25 |
Cancer |
Yes |
Constrictive pericarditis |
No |
|
Yanagi et al. [17] |
2014 |
Japan |
C |
1 |
81 |
M |
Dyspnea, peripheral edema, anorexia |
N/A |
HTN |
Yes |
Thickening, constrictive pericarditis |
No |
|
Matsumiya et al. [18] |
2015 |
Japan |
C |
1 |
50 |
F |
Chest pain, fever, fatigue |
1 |
Asthma |
Yes |
Thickening |
Mediastinal lymph nodes |
|
Mori et al. [19] |
2015 |
Japan |
C |
1 |
65 |
M |
Nausea, abdominal pain |
0.1 |
Dyslipidemia |
No |
Thickening |
Pancreas, biliary system |
|
Sendo et al. [20] |
2015 |
Japan |
C |
1 |
78 |
F |
Dyspnea |
N/A |
HTN, pulmonary tuberculosis, asthma |
No |
Thickening, effusion |
Pancreas, multiple lymph nodes |
|
Horie et al. [21] |
2016 |
Japan |
C |
1 |
73 |
M |
Dyspnea |
2 |
HTN, DM |
Yes |
Thickening, constrictive pericarditis, effusion |
No |
|
Hourai et al. [22] |
2016 |
Japan |
C |
1 |
75 |
M |
Incidental finding |
N/A |
HD |
Yes |
Thickening, effusion |
Mediastinal lymph nodes, coronary artery |
|
Ibe et al. [23] |
2016 |
Japan |
C |
1 |
72 |
M |
Dyspnea, weight loss |
1 |
No |
Yes |
Thickening, constrictive pericarditis, effusion, nodule on the pericardium |
No |
|
Kondo et al. [24] |
2016 |
Japan |
C |
1 |
78 |
M |
Peripheral edema, elevated liver enzymes |
24 |
N/A |
Yes |
Thickening, constrictive pericarditis, |
Pleural thickening/plaques, sclerosing cholangitis |
|
Moreno et al. [25] |
2016 |
Spain |
C |
1 |
70 |
M |
Dyspnea |
12 |
HTN, dyslipidemia, HD, COPD, CML, aortitis |
Yes |
Pericardial effusion |
Aorta, bilateral renal sinus fat |
|
Terzic et al. [26] |
2017 |
Serbia |
C |
1 |
53 |
M |
Fatigue |
12 |
N/A |
No |
Thickening, constrictive pericarditis, effusion, calcification |
Retroperitoneum |
|
Matsuda et al. [27] |
2018 |
Japan |
C |
1 |
70 |
F |
Bilateral lacrimal gland enlargement |
24 |
N/A |
No |
Pericarditis |
Coronary artery, lacrimal glands, retroperitoneum, pancreas and right common iliac artery, ascending aorta |
|
Steiner et al. [28] |
2018 |
USA |
P |
1 |
78 |
M |
Dyspnea, peripheral edema, ascites |
N/A |
N/A |
Yes |
Thickening, constrictive pericarditis, calcification, pericardial mass |
No |
|
Weiss et al. [29] |
2018 |
USA |
C |
1 |
83 |
M |
Incidental finding |
N/A |
Cancer, constrictive pericarditis, pericardial effusion, HD |
No |
Thickening, constrictive pericarditis |
No |
|
Arao et al. [30] |
2019 |
Japan |
C |
1 |
64 |
F |
Abdominal fulness |
1 |
HTN, Asthma |
No |
Pericardial effusion |
Ureteral wall |
|
Gorecka et al. [31] |
2019 |
Ireland |
C |
1 |
53 |
F |
Weight loss, chest pain, fatigue |
6 |
No |
Yes |
Thickening, effusion |
No |
|
Sly et al. [32] |
2019 |
USA |
P |
1 |
37 |
M |
Chest pain |
N/A |
N/A |
No |
Thickening, effusion |
No |
|
Tomoda et al. [33] |
2019 |
Japan |
C |
1 |
72 |
F |
Dyspnea |
N/A |
N/A |
No |
Pericarditis, nodules on pericardium |
Mediastinal lymph nodes |
|
Wang et al. [34] |
2019 |
China |
P |
1 |
80 |
M |
Dyspnea |
3 |
N/A |
Yes |
Thickening, constrictive pericarditis, calcification. |
No |
|
Yassi et al. [35] |
2019 |
USA |
C |
1 |
36 |
M |
Chest pain |
N/A |
No |
No |
Thickening, constrictive pericarditis |
No |
|
Meier et al. [36] |
2020 |
USA |
C |
1 |
78 |
M |
Acute respiratory failure |
N/A |
Recurrent pericardial and pleural effusion |
Yes |
Pericardial effusion |
Aorta, retroperitoneal fibrosis |
|
Yamamoto et al. [37] |
2020 |
Japan |
C |
1 |
75 |
M |
Incidental finding |
N/A |
DM, HD, dementia |
No |
Thickening, pericardial nodule, effusion |
No |
|
Yuriditsky et al. [38] |
2020 |
USA |
C |
1 |
79 |
M |
Dyspnea |
N/A |
HD, recurrent pleural effusion |
Yes |
Thickening, constrictive pericarditis, pericardial calcification |
No |
|
Corona-Rodarte et al. [39] |
2021 |
Mexico |
C |
1 |
44 |
M |
Cough, peripheral edema, dyspnea, weight loss, fever |
4 |
N/A |
Yes |
Constrictive pericarditis, effusion |
No |
|
Doumen et al. [40] |
2021 |
Belgium |
C |
1 |
76 |
F |
Dyspnea, cough |
2 |
DM, HTN, COPD, dyslipidemia |
Yes |
Pericarditis |
No |
|
Fujita et al. [41] |
2021 |
Japan |
C |
1 |
83 |
M |
Dyspnea, weight gain |
0.25 |
N/A |
Yes |
Thickening, constrictive pericarditis |
No |
|
Majid et al. [42] |
2022 |
USA |
C |
1 |
54 |
M |
Dyspnea, peripheral edema, chest pain, fatigue |
N/A |
HTN |
No |
Thickening, constrictive pericarditis |
No |
|
Maltes et al. [43] |
2022 |
Portugal |
C |
1 |
68 |
M |
Peripheral edema, ascites |
N/A |
HD |
No |
Thickening, constrictive pericarditis |
No |
|
Ohman et al. [44] |
2022 |
USA |
P |
1 |
51 |
F |
Jaundice, ascites, weight loss |
N/A |
Asthma |
No |
Thickening, constrictive pericarditis |
Omentum, uterus |
|
George et al. [45] |
2023 |
USA |
P |
1 |
41 |
M |
Vomiting, weight loss, dyspnea |
12 |
N/A |
No |
Thickening, constrictive pericarditis |
No |
|
Kawanami et al. [46] |
2023 |
Japan |
C |
1 |
66 |
M |
Refractory pericarditis |
N/A |
N/A |
Yes |
Thickening, effusion, pericardial nodule |
Aortic root, coronary arteries |
|
Lildar et al. [47] |
2023 |
USA |
P |
1 |
79 |
M |
Dyspnea |
N/A |
Cancer, chronic pancreatitis |
No |
Pericardial effusion |
Pancreas, multiple lymph nodes |
|
Saad et al. [48] |
2023 |
Egypt |
C |
1 |
13 |
F |
Dyspnea, hemoptysis, fever, weight loss |
2 |
N/A |
Yes |
Thickening, effusion |
Lungs |
|
Sugawara et al. [49] |
2023 |
Japan |
C |
1 |
67 |
F |
Chest pain, palpitation |
N/A |
DM, Asthma |
No |
Pericardial effusion |
Submandibular gland |
|
Wei et al. [50] |
2023 |
Japan |
C |
1 |
82 |
F |
Chest pain, abdominal fullness |
8 |
N/A |
Yes |
Pericardial effusion |
Submandibular glands, pharyngeal tonsils |
|
An et al. [51] |
2024 |
Korea |
C |
1 |
66 |
F |
Dyspnea, peripheral edema |
0.5 |
N/A |
Yes |
Thickening, constrictive pericarditis, effusion |
No |
|
Miura et al. [52] |
2024 |
Japan |
C |
1 |
72 |
M |
Incidental finding |
N/A |
N/A |
No |
Thickening, effusion |
Aorta, coronary artery, submandibular gland |
|
Okabe et al. [53] |
2024 |
Japan |
C |
1 |
82 |
M |
Peripheral edema, sialadenitis |
3 |
DM, HD, idiopathic exophthalmos |
Yes |
Constrictive pericarditis, calcification |
Salivary glands, orbit |
|
Ozgur et al. [54] |
2024 |
USA |
C |
1 |
55 |
M |
Dyspnea, peripheral edema, hemoptysis, weight loss |
6 |
DM |
Yes |
Thickening, constrictive pericarditis |
No |
|
Shimada et al. [55] |
2024 |
Japan |
C |
1 |
72 |
M |
Scrotal edema, ascites |
N/A |
HTN |
Yes |
Thickening, constrictive pericarditis |
Aorta |
|
Son et al. [56] |
2024 |
Korea |
C |
1 |
77 |
M |
Anorexia, fever |
3 |
HTN, dyslipidemia |
Yes |
Thickening, effusion |
No |
|
Thummala et al. [57] |
2024 |
USA |
P |
1 |
51 |
M |
Dyspnea |
N/A |
HD, CKD |
No |
Thickening, constrictive pericarditis |
No |
|
Ono et al. [58] |
2025 |
Japan |
C |
1 |
67 |
M |
Dyspnea, peripheral edema, anorexia |
6 |
Liver dysfunction |
Yes |
Thickening, constrictive pericarditis |
No |
| M: Male, F: Female, DM: Diabetes mellitus, HTN: Hypertension, CKD: Chronic liver disease, HD: Heart disease, COPD: Chronic obstructive lung disease, CML: Chronic myeloid leukemia, USA: United States of America, P: Conference abstracts, C: Case report, N/A: Not available. | ||||||||||||
|
Author |
X-ray |
Echocardiography |
CT |
Cardiac MRI |
PET-CT |
ECG |
Right heart catheterization |
|
Khodabandeh et al. [9] |
N/A |
Constrictive physiology, pericardial thickening |
Pericardial thickening, bilateral pleural effusion |
N/A |
N/A |
N/A |
Constrictive physiology |
|
Horie et al. [10] |
Bilateral pleural effusion |
Constrictive physiology |
N/A |
Suggestive of constrictive pericarditis |
N/A |
N/A |
Constrictive physiology |
|
Kabara et al. [11] |
Cardiomegaly |
Collapsed left atrium, pleural effusion |
Pericardial effusion, retroperitoneal fibrosis |
N/A |
N/A |
N/A |
N/A |
|
Sekiguchi et al. [12] |
Bilateral lung opacities |
Constrictive physiology |
Pericardial thickening, bilateral pleural thickening, right-sided pleural effusion |
N/A |
N/A |
N/A |
N/A |
|
Sekiguchi et al. [13] |
Bilateral pleural effusion |
Constrictive physiology |
N/A |
Constrictive physiology |
N/A |
N/A |
Constrictive physiology |
|
Kassier et al. [14] |
N/A |
Pericardial thickening, pericardial effusion, pericardial calcification |
N/A |
Pericardial thickening, pericardial delayed hyperenhancement |
N/A |
Atrial flutter |
Constrictive physiology |
|
Morita et al. [15] |
Cardiomegaly |
Pericardial thickening |
Pericardial thickening |
N/A |
N/A |
Normal sinus rhythm |
N/A |
|
Seo et al. [16] |
Bilateral pleural effusion, cardiomegaly |
Constrictive physiology, pericardial effusion |
N/A |
N/A |
Pericardial effusion |
N/A |
N/A |
|
Yanagi et al. [17] |
Bilateral pleural effusion |
Pericardial thickening, constrictive physiology |
Pericardial thickening |
Constrictive physiology, pericardial thickening |
N/A |
Sinus rhythm, low-voltage QRS complexes |
Constrictive physiology |
|
Matsumiya et al. [18] |
Normal |
Pericardial thickening |
Pericardial thickening, left-sided pleural effusion, mediastinal lymphadenopathy |
N/A |
Patchy uptake in the pericardium, and a dense uptake in the mediastinal lymph node |
Normal sinus rhythm |
N/A |
|
Mori et al. [19] |
Normal |
Normal |
Pericardial thickening, pancreatic parenchyma enlargement, common bile duct wall thickening |
N/A |
N/A |
Normal sinus rhythm |
N/A |
|
Sendo et al. [20] |
Cardiomegaly |
Pericardial effusion |
Pericardial effusion, lymphadenopathies in the mediastinum, para-aorta, abdominal cavity, and inguinal region. diffuse enlargement of the pancreas, and bilateral hydronephrosis. |
N/A |
N/A |
N/A |
N/A |
|
Horie et al. [21] |
Bilateral pleural effusion, cardiomegaly |
Pericardial effusion |
N/A |
N/A |
Localized uptake in the pericardium |
N/A |
Constrictive physiology |
|
Hourai et al. [22] |
Cardiomegaly, bilateral pleural effusion |
N/A |
Pericardial thickening, pericardial effusion, and thickening of the perivascular regions of the abdominal aorta |
N/A |
Enhanced uptake in mediastinal lymph nodes, pericardium, and perivascular regions of the abdominal aorta and aortic wall |
N/A |
N/A |
|
Ibe et al. [23] |
Bilateral pleural effusion |
Constrictive physiology, pericardial effusion |
N/A |
Constrictive physiology, pericardial thickening, pericardial effusion, enhancement of pericardium |
N/A |
N/A |
Constrictive physiology |
|
Kondo et al. [24] |
N/A |
N/A |
N/A |
N/A |
Bilateral pleural effusion, thickening of the pleuro-pericardial wall, and accumulation in the right pleura |
N/A |
Constrictive physiology |
|
Moreno et al. [25] |
N/A |
Pericardial effusion |
Pericardial effusion, bilateral pleural effusion, aortitis, and bilateral obliteration of renal sinus fat |
N/A |
N/A |
N/A |
N/A |
|
Terzic et al. [26] |
Cardiomegaly |
Pericardial effusion |
Pericardial effusion, retroperitoneal fibrosis |
N/A |
N/A |
Sinus rhythm, low-voltage QRS complexes |
N/A |
|
Matsuda et al. [27] |
N/A |
N/A |
Left circumflex artery wall thickening |
N/A |
Uptake in left ventricular wall, left circumflex artery wall, and ascending aorta. e findings suggested dacryoadenitis, retroperitoneal fibromatosis, pancreatic periarteritis, and right common iliac periarteritis. |
N/A |
N/A |
|
Steiner et al. [28] |
N/A |
Constrictive physiology, anterior pericardial mass |
Pericardial thickening, pericardial calcification, pericardial nodule, bilateral pleural effusion |
N/A |
N/A |
N/A |
Not definitive for constrictive physiology |
|
Weiss et al. [29] |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Arao et al. [30] |
Cardiomegaly |
Pericardial effusion |
Pericardial effusion, ureteral wall thickening |
N/A |
N/A |
Sinus rhythm, low-voltage QRS complexes |
N/A |
|
Gorecka et al. [31] |
Bilateral pleural effusion |
Pericardial effusion, mild impairment of left ventricular function and moderate mitral regurgitation |
N/A |
Pericardial thickening, and active inflammation of the pericardium |
N/A |
N/A |
N/A |
|
Sly et al. [32] |
N/A |
Constrictive physiology, pericardial effusion |
Pericardial effusion |
N/A |
N/A |
Diffuse ST elevations and PR depression. |
Constrictive physiology |
|
Tomoda et al. [33] |
N/A |
Pericardial effusion |
Pericardial thickening, mediastinal lymphadenopathy |
N/A |
N/A |
N/A |
N/A |
|
Wang et al. [34] |
N/A |
N/A |
Pericardial thickening, bilateral pleural effusion, ascites |
N/A |
N/A |
N/A |
Constrictive physiology |
|
Yassi et al. [35] |
N/A |
N/A |
N/A |
N/A |
N/A |
Changes consistent with acute pericarditis |
N/A |
|
Meier et al. [36] |
N/A |
N/A |
Pericardial effusion, right-sided pleural effusion, aortitis, pulmonary embolism, retroperitoneal fibrosis. |
N/A |
N/A |
N/A |
N/A |
|
Yamamoto et al. [37] |
N/A |
N/A |
Pericardial thickening, pericardial nodules, and pericardial and paraphrenic lymph node enlargement |
Pericardial thickening, pericardial effusion, with inhomogeneous gadolinium enhancement |
Metabolic activity in the pericardial nodular lesions |
Normal sinus rhythm |
Normal |
|
Yuriditsky et al. [38] |
N/A |
Constrictive physiology |
Pericardial thickening, right-sided pleural effusion |
Constrictive physiology |
N/A |
Sinus rhythm, incomplete right bundle branch block and a left anterior fascicular block |
Constrictive physiology |
|
Corona-Rodarte et al. [39] |
N/A |
Constrictive physiology, pericardial effusion |
Prevascular, and pretracheal mediastinal lymphadenopathies, bilateral pleural effusion, atelectasis, pericardial effusion with contrast enhancement of the pericardium |
Suggestive of constrictive pericarditis |
N/A |
N/A |
N/A |
|
Doumen et al. [40] |
Bilateral pleural effusion, cardiomegaly |
Pericardial effusion |
Normal after treatment |
Pericardial effusion, pericardial thickening |
Unspecified pericardial uptake |
Sinus rhythm, incomplete right bundle branch block |
N/A |
|
Fujita et al. [41] |
Bilateral pleural effusion, cardiomegaly |
Pericardial thickening, constrictive physiology |
Pericardial thickening, pericardial effusion |
Adhesion between pericardium and myocardium |
N/A |
Sinus rhythm, low-voltage QRS complexes |
Constrictive physiology |
|
Majid et al. [42] |
N/A |
Constrictive physiology, pericardial effusion |
N/A |
Pericardial effusion, constrictive physiology, marked pericardial delayed enhancement |
N/A |
N/A |
N/A |
|
Maltes et al. [43] |
N/A |
Pericardial thickening, constrictive physiology |
Pericardial thickening, an anomalous pulmonary venous return |
Pericardial thickening with diffuse late gadolinium enhancement |
N/A |
N/A |
Constrictive physiology |
|
Ohman et al. [44] |
N/A |
Constrictive physiology |
Hepatomegaly, thickening of the uterus, and omentum |
Constrictive physiology, pericardial thickening |
N/A |
N/A |
N/A |
|
George et al. [45] |
N/A |
Pericardial thickening, and myocardial-pericardial tethering of left ventricle apical segments |
Pericardial thickening |
Constrictive physiology |
N/A |
N/A |
Constrictive physiology |
|
Kawanami et al. [46] |
N/A |
Pericardial effusion, a hyperechoic lesion around the aortic root, and multiple nodules in the pericardium |
Pericardial thickening. contrast-enhancing nodules along the pericardium and soft tissue around the aortic root, coronary artery, and dorsal left atrium |
N/A |
Focal uptake consistent with the lesions detected by contrast-enhanced CT |
Normal sinus rhythm |
N/A |
|
Lildar et al. [47] |
N/A |
Pericardial effusion with early diastolic RV collapse, suggestive of cardiac tamponade |
Pancreatic calcification consistent with chronic pancreatitis and fluid localized to the tail suggesting acute uncomplicated pancreatitis |
N/A |
N/A |
Electrical alternans |
N/A |
|
Saad et al. [48] |
N/A |
N/A |
Pericardial thickening, pericardial effusion, left pleural basal thickening in addition to bilateral patchy areas of pulmonary interstitial thickening (consolidation) and ground glass veiling |
N/A |
N/A |
N/A |
N/A |
|
Sugawara et al. [49] |
N/A |
N/A |
Pericardial effusion |
N/A |
Uptake in the pericardium and submandibular gland |
Sinus tachycardia |
N/A |
|
Wei et al. [50] |
N/A |
Pericardial effusion |
Pericardial effusion, bilateral pleural effusion |
N/A |
Pericardial effusion corresponds to an increased area of uptake. Bilateral pharyngeal tonsil, submandibular gland, small lymph nodes in the right side of sternal bone and mediastinum and the wall of ascending aorta |
Sinus rhythm, low T-wave |
N/A |
|
An et al. [51] |
N/A |
Constrictive physiology, pericardial effusion |
Pericardial thickening, pericardial effusion, and left-sided pleural effusions |
N/A |
Patchy uptake in the pericardium |
Sinus rhythm, low-voltage QRS complexes |
N/A |
|
Miura et al. [52] |
N/A |
N/A |
Pericardial thickening, pericardial effusion |
N/A |
N/A |
N/A |
N/A |
|
Okabe et al. [53] |
N/A |
Constrictive physiology |
Pericardial calcification |
N/A |
N/A |
Atrial fibrillation |
Constrictive physiology |
|
Ozgur et al. [54] |
Right-sided pleural effusion. |
Constrictive physiology |
N/A |
Pericardial thickening with increased enhancement on delayed myocardial enhancement |
N/A |
Normal sinus rhythm |
Constrictive physiology |
|
Shimada et al. [55] |
Cardiomegaly, blunted left costophrenic angle |
Constrictive physiology |
Pericardial thickening, DeBakey type II aortic dissection |
N/A |
N/A |
Normal sinus rhythm |
N/A |
|
Son et al. [56] |
Cardiomegaly, blunted left costophrenic angle |
Constrictive physiology, pericardial effusion, fibrinous strands |
Pericardial thickening, pericardial effusion, and bilateral pleural effusions |
N/A |
Diffusely increased uptake in the pericardium |
Sinus tachycardia |
N/A |
|
Thummala et al. [57] |
N/A |
N/A |
N/A |
Constrictive physiology, pericardial thickening |
N/A |
N/A |
Constrictive physiology |
|
Ono et al. [58] |
Cardiomegaly, blunted right costophrenic angle |
Pericardial thickening, constrictive physiology |
Right-sided pleural effusion, pericardial thickening |
N/A |
N/A |
Normal sinus rhythm, prominent P waves |
Constrictive physiology |
| CT: Computed tomography, MRI: Magnetic resonance imaging, PET: Positron emission tomography. | |||||||
|
Author |
Serum IgG4 (mg/dl) |
Serum IgG4/IgG |
Pericardial tissue IgG4/IgG |
Pericardial IgG4/HPF |
Histopathology sample location |
Unsuccessful initial treatment |
Successful treatment |
Maintenance treatment |
Follow-up (months) |
Outcome |
|
Khodabandeh et al. [9] |
150 |
N/A |
N/A |
N/A |
Pericardium |
Thoracentesis |
Pericardiectomy |
N/A |
N/A |
Clinically improved |
|
Horie et al. [10] |
N/A |
N/A |
34% |
33 |
Pericardium |
Diuretics, after-load reducing agent, paracenteses and thoracenteses |
Pericardiectomy |
No |
24 |
Remission |
|
Kabara et al. [11] |
408 |
23% |
N/A |
N/A |
N/A |
No |
Prednisolone |
Prednisolone |
1 |
Remission |
|
Sekiguchi et al. [12] |
136 |
No |
N/A |
N/A |
Pleura |
No |
Prednisone |
Prednisone for 6 months then stopped |
12 |
Remission |
|
Sekiguchi et al. [13] |
N/A |
N/A |
34% |
33 |
Pericardium |
Paracentesis, Thoracocentesis, diuretics, afterload-reducing agents |
Pericardiectomy |
No |
24 |
Remission |
|
Kassier et al. [14] |
62 |
N/A |
"Increased" |
"Increased" |
Pericardium |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Morita et al. [15] |
1800 |
87% |
"Increased" |
"Increased" |
Pericardium |
Pericardiocentesis |
Prednisolone |
Prednisolone |
18 |
Remission |
|
Seo et al. [16] |
150 |
10.18% |
5% |
30 |
Pericardium |
Furosemide, spironolactone, pericardial drainage |
Pericardiectomy |
Prednisolone |
N/A |
Remission |
|
Yanagi et al. [17] |
196 |
No |
68% |
24 |
Pericardium |
No |
Pericardiectomy and pericardiotomy (Waffle procedure) |
Prednisolone |
1.25 |
Remission |
|
Matsumiya et al. [18] |
428 |
N/A |
No |
N/A |
Mediastinal biopsy |
No |
Prednisolone |
N/A |
28 |
Remission |
|
Mori et al. [19] |
637 |
N/A |
N/A |
N/A |
No |
No |
Prednisolone |
Prednisolone |
24 |
Remission |
|
Sendo et al. [20] |
921 |
24.10% |
51% |
86 |
Inguinal lymph node and pericardium |
Pericardiocentesis |
Pericardial drainage, prednisolone |
N/A |
N/A |
Clinically improved |
|
Horie et al. [21] |
122 |
7% |
42% |
N/A |
Pericardium |
Pericardiocentesis, furosemide, tolvaptan, dobutamine |
Prednisolone |
Prednisolone |
2 |
Remission |
|
Hourai et al. [22] |
625 |
18% |
N/A |
N/A |
Mediastinal lymph node |
No |
Corticosteroid |
Corticosteroid |
N/A |
Remission |
|
Ibe et al. [23] |
177 |
8.70% |
>50% |
"Increased" |
Pericardium |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Kondo et al. [24] |
700 |
N/A |
50% |
30 |
Pericardium and pleura |
Prednisolone |
Pericardiectomy |
N/A |
N/A |
Clinically improved |
|
Moreno et al. [25] |
437 |
N/A |
N/A |
N/A |
No |
No |
Methylprednisolone |
Prednisone, cyclophosphamide |
10 |
Remission |
|
Terzic et al. [26] |
163 |
N/A |
N/A |
N/A |
Pericardium |
Ibuprofen, ceftriaxone, prednisolone |
Pericardiectomy, prednisolone, azathioprine |
Prednisolone, azathioprine |
48 |
Several recurrences |
|
Matsuda et al. [27] |
785 |
38.60% |
No |
N/A |
Lacrimal glands |
No |
Prednisolone |
Prednisolone |
2 |
Remission |
|
Steiner et al. [28] |
205 |
No |
"Increased" |
"Increased" |
Pericardium |
Diuretics, anti-inflammatory drugs, thoracentesis |
Pericardiectomy |
N/A |
N/A |
Clinically improved |
|
Weiss et al. [29] |
N/A |
N/A |
No |
>50 |
Pericardial biopsy from 5 years ago |
No |
Pericardiectomy |
No |
60 |
Remission |
|
Arao et al. [30] |
962 |
46.70% |
No |
N/A |
No |
No |
Prednisolone |
Prednisolone |
24 |
Remission |
|
Gorecka et al. [31] |
N/A |
N/A |
>40% |
N/A |
Pericardium |
No |
CD 20 monoclonal antibody |
CD 20 monoclonal antibody |
N/A |
Remission |
|
Sly et al. [32] |
Elevated |
No |
"Increased" |
"Increased" |
Pericardium |
Aspirin, colchicine, pericardiocentesis |
Pericardiectomy |
Prednisone |
N/A |
Remission |
|
Tomoda et al. [33] |
3580 |
56.10% |
No |
N/A |
Mediastinal lymph nodes |
No |
Prednisolone |
Prednisolone |
12 |
Remission |
|
Wang et al. [34] |
N/A |
N/A |
No |
"Increased" |
Pericardium |
No |
Pericardiotomy, prednisone |
Prednisone |
60 |
Remission |
|
Yassi et al. [35] |
Elevated |
No |
65% |
65 |
Pericardium |
Aspirin, colchicine, vancomycin, cefazolin, corticosteroid, ibuprofen |
Pericardial window |
Corticosteroid |
3 |
Recurrence |
|
Meier et al. [36] |
N/A |
N/A |
N/A |
4 |
Pericardium and pleura |
thoracoscopic pleurodesis and tunneled right pleural catheter, pericardiotomy |
Corticosteroid, rituximab |
Corticosteroid, rituximab |
N/A |
Clinically improved |
|
Yamamoto et al. [37] |
212 |
No |
51% |
29 |
Pericardium |
No |
Prednisone |
Prednisolone |
6 |
Remission |
|
Yuriditsky et al. [38] |
306 |
10.70% |
N/A |
"Few" |
Pericardium and pleura |
Prednisone, diuretics |
Pericardiectomy |
Corticosteroid |
2 |
Remission |
|
Corona-Rodarte et al. [39] |
55 |
N/A |
>40% |
N/A |
Pericardium |
Anti-tuberculous antibiotics, corticosteroid. Then after 1 month done pericardiectomy, ceftriaxone, vancomycin |
No |
N/A |
1 |
Dead |
|
Doumen et al. [40] |
179 |
15.70% |
> 80% |
50 |
Pericardium |
Pericardiocentesis, pleural drainage, Colchicine + NSAID |
Pericardiectomy |
No |
8 |
Remission |
|
Fujita et al. [41] |
165 |
No |
50% |
"Increased" |
Pericardium |
Diuretics, dobutamine, and non-invasive positive pressure ventilation, pericardial drainage |
Pericardiectomy |
No |
6 |
Remission |
|
Majid et al. [42] |
N/A |
N/A |
>30% |
N/A |
Pericardium |
Steroid taper, colchicine and ibuprofen |
Pericardiectomy |
N/A |
N/A |
Clinically improved |
|
Maltes et al. [43] |
N/A |
N/A |
N/A |
>20 |
Pericardium |
No |
Pericardiectomy and surgical correction of anomalous pulmonary venous return |
Corticosteroid |
N/A |
Clinically improved |
|
Ohman et al. [44] |
Elevated |
No |
No |
N/A |
Omental biopsy |
N/A |
N/A |
Corticosteroid |
N/A |
N/A |
|
George et al. [45] |
123 |
N/A |
>40% |
>30 |
Pericardium |
No |
Pericardiectomy, prednisone, azathioprine |
Prednisone, azathioprine |
2 |
Remission |
|
Kawanami et al. [46] |
415 |
N/A |
"Increased" |
N/A |
Pericardium |
NSAID, colchicine |
Prednisolone |
N/A |
0.5 |
Remission |
|
Lildar et al. [47] |
422 |
N/A |
N/A |
N/A |
No |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Saad et al. [48] |
168 |
6.40% |
No |
"Increased" |
Pericardium |
Pericardiocentesis, pericardiotomy |
No |
Prednisone, azathioprine, mycophenolate mofetil |
30 |
Dead, massive hemoptysis |
|
Sugawara et al. [49] |
2281 |
59.20% |
N/A |
N/A |
Submandibular gland |
No |
Pericardial drainage, prednisolone |
N/A |
12 |
Remission |
|
Wei et al. [50] |
1400 |
81.40% |
N/A |
N/A |
Submandibular gland |
Furosemide, spironolactone |
Methylprednisolone, mycophenolate mofetil |
Prednisone |
12 |
Remission |
|
An et al. [51] |
2550 |
N/A |
>20% |
>50 |
Pericardium |
No |
prednisolone, colchicine, furosemide |
Prednisolone, colchicine for 3 months, then only prednisolone |
24 |
Remission |
|
Miura et al. [52] |
2270 |
N/A |
N/A |
N/A |
Submandibular gland |
No |
Corticosteroid |
Corticosteroid |
12 |
Remission |
|
Okabe et al. [53] |
1168 |
No |
40% |
10 |
Minor salivary gland, Pericardium |
Diuretics, beta blocker |
Pericardiectomy, waffle procedure, corticosteroid |
Rituximab |
18 |
Remission (improved exophthalmos) |
|
Ozgur et al. [54] |
1216 |
No |
No |
N/A |
Pericardium |
No |
Pericardiectomy |
N/A |
2 |
Remission |
|
Shimada et al. [55] |
263 |
No |
No |
N/A |
Aortic biopsy |
Diuretics, anti-hypertensive drugs |
Ascending aortic replacement, pericardiotomy followed by adhesion debridement |
Diuretic and prednisolone for 6 months, then stopped |
12 |
Remission |
|
Son et al. [56] |
234 |
No |
50% |
>50 |
Pericardium |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Thummala et al. [57] |
Elevated |
No |
No |
"Increased" |
Pericardium |
N/A |
N/A |
N/A |
N/A |
N/A |
|
Ono et al. [58] |
679 |
N/A |
<70 |
70 |
Pericardium |
Diuretics |
Pericardiectomy |
No |
36 |
Remission |
Patient characteristics
A total of 50 patients were included in the study with a mean age of 64.86±15.79 years. There were 36 (72%) males. The past medical histories of 29 patients were provided which included hypertension 9 (31%), heart disease 8 (27.6%), and diabetes mellitus 6 (20.7%). Only two (4%) patients were previously diagnosed with IgG4-RD and one (2%) patient was suspected but not confirmed. No (0%) family history of IgG4-RD was reported in the included studies. The most common symptoms of patients included dyspnea 27 (54%), peripheral edema 16 (32%), chest pain 9 (18%), and weight loss 7 (14%). The mean duration of the presenting complaint was provided in 27 patients which was 9.4 months (Table 4).
|
Variables |
Number (%) |
|
Age (mean ± SD) |
64.86±15.79 |
|
Sex |
|
|
Male |
36 (72) |
|
Female |
14 (28) |
|
Past medical histories (29) |
|
|
HTN |
9 (31) |
|
HD |
8 (27.6) |
|
DM |
6 (20.7) |
|
Dyslipidemia |
4 (13.8) |
|
Asthma |
4 (13.8) |
|
Malignancy |
3 (10.3) |
|
COPD |
2 (6.9) |
|
Pericardial effusion |
2 (6.9) |
|
Pleural effusion |
2 (6.9) |
|
Chronic pancreatitis |
1 (3.4) |
|
Chronic kidney disease |
1 (3.4) |
|
Aortitis |
1 (3.4) |
|
Idiopathic exophthalmos |
1 (3.4) |
|
Liver dysfunction |
1 (3.4) |
|
Pulmonary tuberculosis |
1 (3.4) |
|
Dementia |
1 (3.4) |
|
Presenting symptoms (50) |
|
|
Dyspnea |
27 (54) |
|
Peripheral edema |
16 (32) |
|
Chest pain |
9 (18) |
|
Weight loss |
7 (14) |
|
Fatigue |
5 (10) |
|
Fever |
4 (8%) |
|
Ascites |
4 (8%) |
|
Anorexia |
3 (6%) |
|
Cough |
2 (4%) |
|
Hemoptysis |
2 (4%) |
|
Nausea and Vomiting |
2 (4%) |
|
Abdominal fullness |
2 (4%) |
|
Jaundice |
1 (2%) |
|
Abdominal pain |
1 (2%) |
|
Palpitation |
1 (2%) |
|
Weight gain |
1 (2%) |
|
Sialadenitis |
1 (2%) |
|
Lacrimal gland enlargement |
1 (2%) |
|
Referred for cardiac tamponade |
1 (2%) |
|
Refractory pericarditis |
1 (2%) |
|
Elevated liver enzymes |
1 (2%) |
|
Incidental finding |
4 (8%) |
|
Pericardial manifestations (50) |
|
|
Pericarditis |
50 (100) |
|
Pericardial thickening |
37 (74) |
|
Constrictive pericarditis |
28 (56) |
|
Pericardial effusion |
23 (46) |
|
Pericardial calcification |
6 (12) |
|
Pericardial nodule |
5 (10) |
|
Pleural disease (50) |
|
|
Yes |
30 (60) |
|
No |
20 (40) |
|
Number of organs affected (50) |
|
|
Isolated pericardial involvement |
28 (56) |
|
Pericardium and one other organ |
8 (16) |
|
Pericardium and two other organs |
11 (22) |
|
Pericardium and three other organs |
2 (4) |
|
Pericardium and six other organs |
1 (2) |
|
Extra-pericardial IgG4-RD organ involvement (50) |
|
|
Aorta |
6 (12) |
|
Lymph nodes |
6 (12) |
|
Pancreas |
4 (8) |
|
Coronary artery |
4 (8) |
|
Retroperitoneum |
3 (6) |
|
Submandibular glands |
3 (6) |
|
Biliary system |
2 (4) |
|
Lacrimal gland |
2 (4) |
|
Pleural tissue |
2 (4) |
|
Right common iliac artery |
1 (2) |
|
Renal sinus fat |
1 (2) |
|
Lungs |
1 (2) |
|
Salivary gland |
1 (2) |
|
Orbit |
1 (2) |
|
Omentum |
1 (2) |
|
Uterus |
1 (2) |
|
Parotid gland |
1 (2) |
|
Pharyngeal tonsils |
1 (2) |
|
Ureteral wall |
1 (2) |
| * The number in parentheses indicates the number of patients for whom information was provided. | |
Pericardial involvement
All patients had pericarditis but with different associated pericardial involvements including pericardial thickening 37 (74%), constrictive pericarditis 28 (56%), and pericardial effusion 23 (46%) (Table 4).
ECG findings
The most common ECG findings were sinus rhythm 7 (30.4%), and sinus rhythm with low voltage QRS complexes 5 (21.7%) (Table 5).
|
Variables |
Number (%) |
|
X-ray findings (22) * |
|
|
Bilateral pleural effusion |
10 (45.5) |
|
Cardiomegaly |
13 (59.1) |
|
Unilateral pleural effusion |
4 (18.2) |
|
Normal |
2 (9.1) |
|
Bilateral lung opacities |
1 (4.5) |
|
Transthoracic echocardiography (38) |
|
|
Constrictive physiology |
21 (55.3) |
|
Pericardial thickening |
9 (23.7) |
|
Pericardial effusion |
19 (50) |
|
Pericardial calcification |
1 (2.6) |
|
Pericardial mass |
1 (2.6) |
|
Fibrinous strands |
1 (2.6) |
|
Cardiac tamponade |
1 (2.6) |
|
Multiple pericardial nodules |
1 (2.6) |
|
Myocardial-pericardial tethering of- left ventricle apical segments |
1 (2.6) |
|
Collapsed left atrium |
1 (2.6) |
|
Moderate mitral regurgitation |
1 (2.6) |
|
Hyperechoic aortic root lesion |
1 (2.6) |
|
Normal |
1 (2.6) |
|
CT findings (37) |
|
|
Pericardial thickening |
22 (59.5) |
|
Pericardial effusion |
16 (43.2) |
|
Pericardial calcification |
2 (5.4) |
|
Pericardial nodule |
3 (8.1) |
|
Bilateral pleural effusion |
7 (18.9) |
|
Unilateral pleural effusion |
6 (16.2) |
|
Retroperitoneal fibrosis |
3 (8.1) |
|
Aorta enhancement |
3 (8.1) |
|
Coronary artery enhancement/thickening |
2 (5.4) |
|
Mediastinal lymphadenopathy |
5 (13.5) |
|
Pulmonary embolism |
1 (2.7) |
|
An anomalous pulmonary venous return |
1 (2.7) |
|
Bilateral renal sinus fat obliteration |
1 (2.7) |
|
Ascites |
1 (2.7) |
|
Pleural thickening |
2 (5.4) |
|
Aortic dissection |
1 (2.7) |
|
Thickening of the uterus |
1 (2.7) |
|
Thickening of omentum |
1 (2.7) |
|
Hepatomegaly |
1 (2.7) |
|
Bilateral pulmonary interstitial thickening |
1 (2.7) |
|
Pancreatic calcification |
1 (2.7) |
|
Enlargement of pancreas |
2 (5.4) |
|
Bile duct wall thickening |
1 (2.7) |
|
Contrast enhancement of- the pericardium, and atelectasis |
1 (2.7) |
|
Abdominal aorta perivascular area thickening |
1 (2.7) |
|
Abdominal lymphadenopathy |
1 (2.7) |
|
Bilateral hydronephrosis |
1 (2.7) |
|
Ureteral wall thickening |
1 (2.7) |
|
Normal |
1 (2.7) |
|
Cardiac MRI findings (17) |
|
|
Constrictive physiology |
11 (64.7) |
|
Pericardial thickening |
10 (58.8) |
|
Pericardial effusion |
4 (23.5) |
|
Pericardial enhancement |
10 (58.8) |
|
PET scan findings (13) |
|
|
Focal pericardial 18F-FDG uptake |
7 (53.8) |
|
Diffuse pericardial 18F-FDG uptake |
3 (14.3) |
|
Unspecified pericardial uptake |
3 (14.3) |
|
Uptake in other organs |
6 (46.1) |
|
ECG findings (23) |
|
|
Normal sinus rhythm |
7 (30.4) |
|
Sinus rhythm, low voltage QRS complexes |
5 (21.7) |
|
Sinus tachycardia |
2 (8.7) |
|
Atrial fibrillation |
1 (4.3) |
|
Atrial flutter |
1 (4.3) |
|
Normal sinus rhythm, prominent P waves |
1(4.3) |
|
Changes consistent with acute pericarditis |
1 (4.3) |
|
Diffuse ST elevations and PR depression. |
1 (4.3) |
|
Electrical alternans |
1 (4.3) |
|
Sinus rhythm, incomplete right bundle branch block |
1 (4.3) |
|
Sinus rhythm, incomplete right bundle-branch block and a left anterior fascicular block |
1 (4.3) |
|
Sinus rhythm, low T-wave |
1 (4.3) |
| * The number in parentheses indicates the number of patients for whom information was provided. | |
Echocardiography findings
Transthoracic echocardiography was done in 38 individuals and revealed constrictive physiology, pericardial effusion, and pericardial thickening in 21 (55.3%), 19 (50%), and 9 (23.7%) patients respectively (Table 5). The ejection fraction was stated in 16 individuals which was normal in 10 (62.5%) and below the normal range in 6 (37.5%).
X-ray findings
Chest x-ray was performed in 22 patients which revealed bilateral pleural effusion in nine (40.9%) of them, and cardiomegaly in 13 (59.1%) patients (Table 5).
CT findings
Computed tomography was conducted in 37 patients. Pericardial thickening and pericardial effusion were detected on CT among 22 (59.5%), and 16 (43.2%) patients respectively. Involvement of organs other than the pericardium included the following: retroperitoneal fibrosis 3 (8.1%), enlargement of the pancreas 2 (5.4%), bile duct thickening 1 (2.7%), mediastinal lymphadenopathy 5 (13.5%), and enhancement of the aorta 3 (8.1%) (Table 5).
Cardiac MRI findings
A cardiac MRI that was performed on 17 individuals, revealed constrictive physiology 11 (64.7%), pericardial thickening 10 (58.8%), and pericardial enhancement 10 (58.8%) among the patients (Table 5).
PET-CT scan findings
Positron emission tomography-CT was performed in 13 patients. Focal pericardial uptake of FDG was present in seven (53.8%) patients, and diffuse uptake in three (14.3%). Involvement of other organs was shown in six individuals (Table 5).
Right heart catheterization findings
Right heart catheterization was performed in 20 patients, revealing constrictive physiology in 18 (90%) individuals. One (5%) patient had no definitive evidence of constrictive physiology, while another (5%) had normal findings.
Laboratory findings
Serum IgG4 levels were reported in 42 patients with their levels being elevated (IgG4> 135mg/dl) in 38 (90.5%) individuals. The exact value of serum IgG4 was given in 38 individuals with a mean of 703.9 mg/dl ± 813.8. The serum ratio of IgG4/IgG was reported in 15 cases with a mean of 32.9% (6.4%-87%).
C-reactive protein was measured in 24 patients, with 19 (79.2%) patients showing elevated levels. The exact value of CRP was reported in 18 individuals with a mean of 61 mg/L.
NT-proBNP was reported in four patients with a mean of 782.45 pg/ml (223 - 15252 pg/ml). and BNP was reported in five patients with a mean of 228.75 pg/ml (10 - 649 pg/ml).
Organs Affected by IgG4-RD
In 28 (56%) patients, only the pericardium was affected. In addition to the pericardium, eight (16%) patients had one other organ affected, eleven (22%) patients had two additional organs affected, two (4%) patients had three additional organs affected, and one (2%) patient had six other organs affected. The organs that are affected are summarized (Table 4).
Diagnosis of IgG4-RD pericarditis
Pericardial tissue IgG4/IgG
Pericardial IgG4 staining was performed in 32 patients. Of these, 25 studies reported the pericardial IgG4/IgG ratio. In 15 (60%) cases, the IgG4/IgG ratio was greater than 40. Five (20%) cases reported an "increased" ratio, although the specific value was not provided. Regarding the number of IgG4/HPF. It was reported in 27 cases of which 25 (92.6%) had more than 10/HPF. These findings along with the combination of IgG4/IgG ratio and IgG4/HPF are summarized (Table 6).
|
Variables |
Number (%) |
|
Pericardial tissue IgG4/IgG ratio (25) * |
|
|
IgG4/IgG >40% |
15 (60) |
|
IgG4/IgG <40% |
5 (20) |
|
“increased” |
5 (20) |
|
Pericardial IgG4+ cells/ HPF (27) |
|
|
1-9/HPF |
2 (7.4) |
|
10-19/HPF |
1 (3.7) |
|
20-29/HPF |
3 (11.1) |
|
30-39/HPF |
5 (18.5) |
|
40-49/HPF |
0 (0) |
|
50>/HPF |
7 (25.9) |
|
“Increased” |
9 (33.3) |
|
Diagnostics for patients with IgG4 staining (32) |
|
|
Pericardial IgG4/IgG ratio> 40%/ “increased” or IgG4/HPF>10/ “increased” |
29 (90.6) |
|
Pericardial IgG4/IgG ratio> 40%/ “increased” and IgG4/HPF>10/ “increased” |
16 (50) |
|
Neither |
3 (9.4) |
|
Methods of diagnosis in those who did not fulfil the above criteria (21) |
|
|
submandibular gland IgG4 staining + CT pericardial finding + increased pericardial uptake on FDG-PETCT + elevated serum IgG4 |
2 (9.5) |
|
Submandibular gland IgG4 staining + CT pericardial findings + elevated serum IgG4 |
1 (4.8) |
|
lacrimal glands IgG4 staining + increased pericardial uptake on FDG-PETCT + elevated serum IgG4 |
1 (4.8) |
|
mediastinal lymph node IgG4 staining + CT pericardial finding + increased pericardial uptake on FDG-PETCT + elevated serum IgG4 |
1 (4.8) |
|
mediastinal lymph node IgG4 staining + CT pericardial findings + elevated serum IgG4 |
1 (4.8) |
|
mediastinal biopsy IgG-4 staining + CT pericardial findings + increased pericardial uptake on FDG-PETCT + elevated serum IgG4 |
1 (4.8) |
|
aortic biopsy IgG4 staining + CT pericardial findings + elevated serum IgG4 |
1 (4.8) |
|
pleural biopsy IgG4 staining + CT pericardial findings + elevated serum IgG4 |
1 (4.8) |
|
Omental biopsy IgG4 staining + elevated serum IgG4 |
1 (4.8) |
|
Pericardial biopsy + Cardiac MRI findings + elevated serum IgG4. |
1 (4.8) |
|
Pericardial biopsy + CT pericardial finding + elevated serum IgG4 |
1 (4.8) |
|
CT retroperitoneum fibrosis + CT pericardial findings + Pericardial biopsy + elevated serum IgG4 |
1 (4.8) |
|
CT aortitis and bilateral obliteration of renal sinus fat + CT pericardial findings + elevated serum IgG4 |
1 (4.8) |
|
CT retroperitoneal fibrosis and aortitis + pericardial biopsy after steroid therapy |
1 (4.8) |
|
Pericardial and pleural biopsy + elevated serum IgG4 |
1 (4.8) |
|
Pericardial biopsy findings + pericardial >30% IgG4- staining + Cardiac MRI findings
|
1 (4.8) |
|
CT ureteral wall thickening + pericardial effusion + elevated serum IgG4 |
1 (4.8) |
|
CT chronic pancreatitis + pericardial effusion + elevated serum IgG4 |
1 (4.8) |
|
CT pericardial fluid analysis + CT retroperitoneal fibrosis + elevated pericardial fluid IgG4 cells + elevated serum IgG4 |
1 (4.8) |
|
CT pericardial finding+ CT pancreatic and biliary system findings + elevated serum IgG4 |
1 (4.8) |
| * The number in parentheses indicates the number of patients for whom information was provided. | |
Methods of diagnosing IgG4 in patients without pericardial biopsy.
In 21 patients who either did not undergo IgG4 staining on their pericardial biopsy or did not have a pericardial biopsy performed, the diagnosis was supported by evidence from biopsies of other organs, elevated serum IgG4 levels, and diagnostic imaging. These findings were consistent with IgG4-related disease as the most likely diagnosis, with no other condition being as probable (Table 6).
Therapeutic approaches and Outcomes
The initial unsuccessful, successful, and maintenance treatment interventions are summarized (Table 7). The outcomes of 44 patients were reported. Remission or clinical improvement was achieved in 40 (90.1%) individuals, recurrence in two (4.5%), and death in two (4.5%) patients. The follow-up period was specified in 33 cases with a mean of 16.3 months (0.5 - 60 months).
|
Variables |
Number (%) |
|
Successful treatment approaches remission or initial clinical improvement (42) * |
|
|
Corticosteroid alone |
14 (33.3) |
|
Corticosteroid + azathioprine + pericardiectomy |
2 (4.8) |
|
Corticosteroid + pericardiectomy + waffle procedure |
1 (2.4) |
|
Corticosteroid + pericardiotomy |
1 (2.4) |
|
Corticosteroid + pericardial drainage |
2 (4.8) |
|
Corticosteroid + mycophenolate mofetil |
1 (2.4) |
|
Corticosteroid + colchicine + furosemide |
1 (2.4) |
|
Corticosteroid + rituximab |
1 (2.4) |
|
Pericardiectomy |
14 (33.3) |
|
Pericardiectomy + pericardiotomy + waffle procedure |
1 (2.4) |
|
pericardiectomy and surgical correction of anomalous pulmonary venous return |
1 (2.4) |
|
Pericardial window |
1 (2.4) |
|
Pericardiotomy + ascending aortic replacement |
1 (2.4) |
|
CD-20 monoclonal antibody |
1 (2.4) |
|
Initial unsuccessful approaches (24) |
|
|
Corticosteroid + pericardiectomy + antibiotic |
1 (4.1) |
|
Corticosteroid + NSAID + colchicine + antibiotic |
1 (4.1) |
|
Corticosteroid + NSAID + antibiotic |
1 (4.1) |
|
Corticosteroid |
1 (4.1) |
|
Corticosteroid + diuretic |
1 (4.1) |
|
Corticosteroid + NSAID + colchicine |
1 (4.1) |
|
Pericardiocentesis + NSAID + colchicine |
2 (8.4) |
|
Pericardiocentesis + diuretic |
3 (12.5) |
|
Pericardiocentesis |
2 (8.4) |
|
Pericardiocentesis + pericardiotomy |
1 (4.1) |
|
Diuretics |
4 (16.7) |
|
Diuretics + NSAID + thoracentesis |
1 (4.1) |
|
Diuretic + beta blocker |
1 (4.1) |
|
NSAID + colchicine |
1 (4.1) |
|
Diuretics + thoracentesis |
1 (4.1) |
|
Thoracentesis |
1 (4.1) |
|
Pericardiotomy + thoracoscopic pleurodesis |
1 (4.1) |
|
Maintenance treatment (35) |
|
|
Corticosteroid |
21 (60) |
|
Corticosteroid + Azathioprine |
2 (5.7) |
|
Corticosteroid + Azathioprine + mycophenolate mofetil |
1 (2.9) |
|
Corticosteroid + cyclophosphamide |
1 (2.9) |
|
Corticosteroid + colchicine |
1 (2.9) |
|
Corticosteroid + CD 20 monoclonal antibody |
1 (2.9) |
|
CD 20 monoclonal antibody |
2 (5.7) |
|
Did not receive treatment |
6 (17) |
| * The number in parentheses indicates the number of patients for whom information was provided. | |
Discussion
The tendency of IgG4-RD to affect certain organs has been recognized since the disease was first described. However, consistent patterns of clinical manifestations were not fully evaluated until recently. Currently, the disease is described as having four phenotypes based on the organs affected which include; pancreato-hepatobiliary disease (31%), retroperitoneal fibrosis with or without aortitis (24%), head and neck-limited disease (24%), and classic Mikulicz's syndrome with systemic involvement (22%). These phenotypes are observed to have different demographic characteristics and responses to treatment [4]. Unlike most autoimmune diseases, which predominantly affect females, IgG4-RD causing pericarditis primarily affects males, with an average onset in the sixth decade of life [59-61]. Additionally, most reported cases of IgG4-RD causing pericarditis are reported from Japan, a pattern similar to that observed in Takayasu arteritis. This geographic disparity suggests an underlying genetic predisposition, environmental triggers more commonly found in certain regions, or a higher level of physician awareness and diagnosis. Further research is needed to elucidate the reasons behind this observation [59, 62-65].
In the present study, there were only three patients who reported a history of malignancy, but the follow-up period was not long enough to show if it could have been a premalignant condition and till now there is no definitive research determining the relationship between malignancy and IgG4-RD [1]. However, since the majority of retroperitoneal masses are malignant, and IgG4-RD can present as a mass in the retroperitoneum; it can be initially misdiagnosed as malignancy [66]. For instance, in a study by Zhou et al. which retrospectively examined a group of 63 patients, nearly 60% were initially suspected to have cancer which included lymphoma, pancreatic, colorectal, and gastric cancers, cholangiocarcinoma, and renal cell carcinoma. Alarmingly, some of these patients underwent invasive procedures such as nephrectomy, Whipple procedure, resecting and reconstructing the bile duct, and retroperitoneal mass removal [67].
The most common clinical manifestations in patients were dyspnea, chest pain, and peripheral edema. However, there was significant variability in symptoms, reflecting the disease's ability to affect multiple organs throughout the body. This multisystem involvement contributes to diagnostic complexity. However, such challenges are not unique to IgG4-RD; for instance, Crohn’s disease exhibits extraintestinal manifestations in up to 25% of cases. The presence of non-caseating granulomatous inflammation aids in differentiating Crohn’s disease. Furthermore, Deshpande et al. have characterized IgG4-RD as analogous to sarcoidosis due to its propensity for multi-organ involvement, while also highlighting shared histopathologic features across affected tissues, Nevertheless, additional research is required to clarify the specific characteristics of the various organs involved in IgG4-RD [2, 68].
Pericardial involvement in IgG4-RD presents with various manifestations, including pericardial effusion, calcification, nodule formation, and pericardial thickening. These differences may be attributed to the stage of disease at which pericarditis was investigated and diagnosed, as the duration of symptoms before clinical presentation is typically prolonged, often spanning months, due to the insidious nature of the condition. This variability could also reflect different phenotypes of IgG4-RD affecting the pericardium, resulting in distinct combinations of the aforementioned pericardial manifestations. However, further research is necessary to validate these hypotheses.
The aorta, coronary artery, and retroperitoneum were the commonly involved “extra-pericardial” organ involvement in this review, which aligns with the manifestation of the retroperitoneal/aortitis phenotype as mentioned by Lanzillotta et al [4]. However, involvement of the pancreas, biliary system, submandibular, and lacrimal glands amongst others were still reported which belongs to the other phenotypes. Thus, it is challenging to determine whether pericarditis is specific to a particular phenotype of IgG4-RD or if it represents a shared manifestation across different phenotypes.
In 2019, the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) collaboratively established classification criteria for IgG4-RD. To satisfy the classification criteria, patients must first show involvement (clinical, radiological, or histopathological) of a classical organ affected by IgG4-RD, which includes the pancreas, kidneys, orbits, aorta, retroperitoneum, major salivary glands, thyroid gland, lacrimal glands, biliary tree, meninges, and paravertebral soft tissue. Furthermore, it should not meet any of the exclusion criteria outlined in detail. However, not all published studies on IgG4-RD fulfill these criteria, nor the ones causing pericarditis were all in line with the classification criteria, which is a limitation of this systematic review, as it adopts a more inclusive approach. Nonetheless, these classification criteria are not intended for clinical diagnostic purposes but rather for ensuring the highest specificity and serve as a useful framework for clinicians when assessing the diagnosis [1, 69].
In regards to laboratory investigations, the serum IgG4 concentration is the most prominent and frequently utilized biomarker for IgG4-RD. Notably, it is elevated in up to 90% of affected individuals. Consistent with this, the present study showed that 90.5% of the patients had elevated serum IgG4 levels. However, this prevalence is shown to vary significantly depending on the patient population studied, with some estimates suggesting elevations in as few as 50% of cases. Serum IgG4 levels can decrease significantly following treatment compared to pre-treatment levels. Although measuring serum IgG4 concentration is often essential for evaluating and managing IgG4-RD over time, its clinical utility should be interpreted within the broader context of the patient’s overall clinical presentation and disease characteristics. In addition, several other biomarkers are associated with disease activity and the extent of organ involvement. Among these, elevated eosinophil counts, increased serum IgG1 and IgE levels, and alterations in serum complement levels have been frequently observed. These markers, in conjunction with serum IgG4, provide valuable insights into disease progression and severity [1]. For example, Gorecka et al. reported increased eosinophil counts, while Matsumiya et al. identified significantly high IgE levels of 1765 IU/ml [18, 31]. Elevated IgE levels were also noted in several other cases of IgG4-RD pericarditis, all of whom had no prior history of allergic reactions [19, 37, 54]. Additionally, ESR was elevated in a subset of patients, as reported by Terzic et al., Moreno et al., and Wei et al [26, 25, 50]. However, the aforementioned biomarkers were not measured or reported in the majority of the included studies, limiting the ability to establish a clear association between them and IgG4-RD pericardial involvement.
While Katz et al. indicated that elevated CRP levels are less commonly observed compared to ESR, high CRP levels were found in a considerable subset of patients with IgG4-RD pericarditis. However, its diagnostic value in IgG4-RD is not well elucidated as CRP can be increased in a myriad of conditions including, immune-mediated diseases, malignancy, and COVID-19 among other communicable diseases [1, 70-73].
The cutoff value of IgG4/HPF for diagnostic purposes varies significantly across different tissues, as reported by Dashpande et al. For biopsy specimens of the kidney and pancreas, it is often greater than 10 IgG4/HPF, whereas in aorta or pleura specimens, it may reach as high as 50 IgG4/HPF. However, there is no established diagnostic cutoff for pericardial tissue, and further research is needed to determine this value. Additionally, given the invasive nature of pericardial biopsy, it may be necessary to rely on biopsies from other sites or diagnostic imaging to reach a diagnosis in clinical practice. Some researchers have suggested a cutoff value of 40% for the IgG4/IgG plasma cell ratio as a general threshold across various organ tissues. While histopathology is crucial for diagnosis, it is important to recognize its limitations. Specifically, sampling errors may result in the absence of all classic histopathological features in certain patients. This is particularly true when small biopsy samples are obtained through CT-guided techniques, as opposed to larger specimens obtained through surgical resection. Such limitations may impact the diagnostic yield of using the IgG4/IgG ratio to assess pericarditis in IgG4-RD. For instance, in the current study, only half of the cases reported an IgG4/IgG ratio for pericardial tissue, with 80% of these showing elevated levels. Nevertheless, IgG4-RD was still considered the most likely cause of pericarditis in all the included patients by relying on other diagnostic methods. If a more stringent inclusion criterion had been applied in this review, a higher specificity might have been achieved. Nonetheless, we believe this study highlights an underreported and often overlooked cause of pericarditis, with histopathological examination being infeasible clinically in most cases due to the risk-benefit ratio [2]. The reported studies relied on several diagnostic imaging methods, for instance, Meier et al. reported that CT showed retroperitoneal fibrosis along with aortitis and the pericardial findings which hinted at the diagnosis of IgG4-RD [36]. Furthermore, Matsuda et al. employed PET scanning, which revealed dacryoadenitis, retroperitoneal fibromatosis, pancreatic periarteritis, and periarteritis of the right common iliac artery, along with uptake in the aorta and coronary artery [27]. The current study revealed the pattern of pericardial uptake on the PET scan varied, ranging from focal to diffuse, potentially indicating different phenotypes of IgG4-RD causing pericarditis or different stages of the same phenotype.
An intriguing case study by Weiss et al. highlights the underdiagnosis of IgG4 as a cause of pericarditis and demonstrates how easily it can be overlooked if there is not a high index of suspicion. The authors reported an 84-year-old man with a history of constrictive pericarditis, for which he had undergone pericardiectomy. However, five years later, the patient presented with an enlarged aorta and pulmonary consolidation. Upon staining the five-year-old pericardial biopsy for IgG4, the results showed an IgG4/HPF count greater than 50. This led to a retrospective diagnosis of constrictive pericarditis due to IgG4-RD [29].
Regarding therapeutic approaches, there was significant variability in treatment combinations for IgG4-RD pericarditis, underscoring the absence of a consensus on managing this condition. Treatment strategies ranged from conservative approaches, with most patients responding well to corticosteroids, to more invasive interventions such as pericardiectomy, which was deemed necessary in a substantial number of patients to achieve clinical improvement, particularly when constrictive physiology was confirmed through right heart catheterization. Maintenance treatment included corticosteroids and other immunosuppressive treatments such as azathioprine, cyclophosphamide, and rituximab. Clinical improvement and remission were achieved in most cases; however, recurrences were still reported in this review. Two patients died, one of whom was reported by Sad et al. In this case, a 13-year-old girl was treated with corticosteroids, azathioprine, mycophenolate mofetil, and rituximab for maintenance to prevent relapses. Unfortunately, these efforts were unsuccessful, as the patient experienced several recurrences and ultimately passed away due to massive hemoptysis, resulting from pleuropericardial and lung involvement of IgG4-RD [48]. In the case reported by Corona-Rodarte et al., a 44-year-old man was undergoing maintenance treatment with corticosteroids for a confirmed diagnosis of IgG4 pericarditis. The patient later presented with dyspnea and fever where his condition worsened, and blood cultures revealed the presence of non-typhoidal Salmonella group D. Subsequently, he developed septic shock and passed away [39].
Even though pericardiectomy was performed in a substantial number of patients, this may be due to the fact that patients who do not respond to non-steroidal anti-inflammatory drugs (NSAIDs) and colchicine for idiopathic pericarditis are typically treated with corticosteroids and are then diagnosed with idiopathic pericarditis [74,75]. Among these patients, those who respond to corticosteroids may actually have IgG4-RD as the underlying cause. However, they may not undergo extensive diagnostic workup to suspect or confirm IgG4-RD. As a result, those who undergo more extensive diagnostic methods are more likely to be diagnosed with this condition, potentially influencing the current available data.
One of the limitations of this systematic review lies in the quality of the case reports, which often exhibit inconsistent reporting of patients' medical histories, medication dosages, and outcomes. In some cases, the focus was solely on the diagnosis of the condition, with no follow-up on treatment results. Additionally, not all patients underwent pericardial IgG4 staining, and in those that did, the IgG4/IgG ratio and IgG4/HPF were not consistently reported, or a specific number was not provided, with some cases merely referring to the levels as "increased." However, a specific cutoff for IgG4 in the pericardium has yet to be agreed upon. Furthermore, serum IgG4 and other biomarkers associated with IgG4-RD were either not performed or not reported in the cases, nor were post-treatment levels of these markers included. Therefore, we strongly encourage the reporting of more detailed case studies, as well as the possibility of a retrospective study on pericardial biopsies to assess the sensitivity and specificity of IgG4 levels in pericardial tissues.
Conclusion
Recognizing IgG4-RD as a potential cause of pericarditis is crucial, as it is often overlooked. It can result in pericardial thickening, pericardial effusion, constrictive pericarditis, and pericardial nodule formation. Corticosteroids and pericardiectomy may result in good outcomes.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: DH was involved in the literature review, study design, and writing of the manuscript. SAB, AM, AG, MAR, MA, KG, GS, RK, and MS were major contributors to the study's conception and involved in the literature review, the study's design, the critical revision of the manuscript. DH and AG confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Use of AI: ChatGPT-4.0 was used to assist in language editing and improving the clarity of the manuscript. All content was reviewed and verified by the authors. Authors are fully responsible for the entire content of their manuscript.
Data availability statement: Not applicable.
References
- Katz G, Stone JH. Clinical perspectives on IgG4-related disease and its classification. Annual review of medicine. 2022;73(1):545-62 doi:10.1146/annurev-med-050219-034449
- Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Modern pathology. 2012;25(9):1181-92. doi:10.1038/modpathol.2012.72
- Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, et al. A new clinicopathological entity of IgG4-related autoimmune disease. Journal of gastroenterology. 2003;38:982-4. doi:10.1007/s00535-003-1175-y
- Lanzillotta M, Mancuso G, Della-Torre E. Advances in the diagnosis and management of IgG4 related disease. Bmj. 2020;369. doi:10.1136/bmj.m1067
- Wallwork R, Perugino CA, Fu X, Harkness T, Zhang Y, Choi HK, et al. The association of smoking with immunoglobulin G4–related disease: a case–control study. Rheumatology. 2021;60(11):5310-7. doi:10.1093/rheumatology/keab172
- Terao C, Ota M, Iwasaki T, Shiokawa M, Kawaguchi S, Kuriyama K, et al. IgG4-related disease in the Japanese population: a genome-wide association study. The Lancet Rheumatology. 2019;1(1):e14-22. doi:10.1016/S2665-9913(19)30006-2
- Perugino CA, Stone JH. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nature Reviews Rheumatology. 2020;16(12):702-14. doi:10.1038/s41584-020-0500-7
- Abdullah HO, Abdalla BA, Kakamad FH, Ahmed JO, Baba HO, Hassan MN, et al. Predatory Publishing Lists: A Review on the Ongoing Battle Against Fraudulent Actions. Barw Med Jour. 2024;2(2):26-30 doi:10.58742/bmj.v2i2.91
- Khodabandeh A, Shadchehr S, Beamis JF. Hyper-IgG4 Disease Presenting As Refractory Pleural Effusion Secondary To Constrictive Pericarditis. InA3. Fellow Case Conference, Fourth Edition 2010 (pp. A6865-A6865). American Thoracic Society. doi:10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A6865
- Horie R, Suri RM, Yi ES, Ryu JH, Sekiguchi H. IgG4-Related Constrictive Pericarditis. InD55. From The White Elephant To The Common Canary: Presentation And Outcomes Of Common And Not-So-Common Critical Illnesses 2012 (pp. A5949-A5949). American Thoracic Society. doi:10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A5949
- Kabara M, Nakagawa N, Chinda J, Hirai T, Nimura A, Ota H, et al. Diagnosis of IgG4-related systemic disease by cytology of large pericardial effusion with fine needle aspiration. International journal of cardiology. 2011;148(3):392-3. doi:10.1016/j.ijcard.2010.12.057
- Sekiguchi H, Horie R, Suri RM, Yi ES, Ryu JH. Constrictive pericarditis caused by immunoglobulin G4-related disease. Circulation: Heart Failure. 2012;5(2):e30-1. doi:10.1161/CIRCHEARTFAILURE.111.966408
- Sekiguchi H, Horie R, Utz JP, Ryu JH. IgG4-related systemic disease presenting with lung entrapment and constrictive pericarditis. Chest. 2012;142(3):781-3. doi:10.1378/chest.11-2608
- Kassier A, Atallah PC. Immunoglobulin G4-Related Disease Constrictive Pericarditis With Pericardial Effusion: A Novel Entity. Journal of the American College of Cardiology. 2014;63(12S):A710. doi:10.1016/S0735-1097(14)60710-5
- Morita T, Izawa A, Hamano H, Asano N, Kozuka A, Moteki H, et al. Significant Pericardial Involvement of Immunoglobulin G4–Related Disease. The Annals of Thoracic Surgery. 2014;98(2):e47-9. doi:10.1016/j.athoracsur.2014.04.069
- Seo J, Song IJ, Lee S, Jeong HJ, Kim HM, Koh BS, et al. A case of constrictive pericarditis due to immunoglobulin G4-related disease. Korean circulation journal. 2015;45(2):161-4. doi:10.4070/kcj.2015.45.2.161
- Yanagi H, Yamazaki I, Shimizu S, Himeno H, Suzuki SI, Masuda M. Constrictive pericarditis caused by immunoglobulin G4–related disease. The Annals of thoracic surgery. 2014;97(3):e71-4. doi:10.1016/j.athoracsur.2013.10.085
- Matsumiya R, Hosono O, Yoshikawa N, Uehara M, Kobayashi H, Oda A, et al. Elevated serum IgG4 complicated by pericardial involvement with a patchy 18F-FDG uptake in PET/CT: atypical presentation of IgG4-related disease. Internal Medicine. 2015;54(18):2337-41. doi:10.2169/internalmedicine.54.4340
- Mori K, Yamada K, Konno T, Inoue D, Uno Y, Watanabe M, et al. Pericardial involvement in IgG4-related disease. Internal Medicine. 2015;54(10):1231-5. doi:10.2169/internalmedicine.54.3856
- Sendo S, Saegusa J, Morinaga Y, Kawakami F, Kogata Y, Kageyama G, et al. IgG4-related disease manifesting as pericarditis with elevated adenosine deaminase and IL-10 levels in pericardial fluid. Modern Rheumatology. 2017;27(5):894-7. doi:10.3109/14397595.2015.1039628
- Horie K, Tada N, Yamaguchi K, Inazawa K, Endo M, Inoue N. Immunoglobulin G4-related constrictive pericarditis identified by cytological examination of pericardial effusion: a case report. Journal of Medical Case Reports. 2016;10:1-5. doi:10.1186/s13256-016-1159-1
- Hourai R, Miyamura M, Tasaki R, Iwata A, Takeda Y, Morita H, et al. A case of IgG4-related lymphadenopathy, pericarditis, coronary artery periarteritis and luminal stenosis. Heart and vessels. 2016;31:1709-13. doi:10.1007/s00380-016-0794-1
- Ibe T, Nakamura T, Taniguchi Y, Momomura SI. IgG4-related effusive constrictive pericarditis. European Heart Journal–Cardiovascular Imaging. 2016;17(6):707. doi:10.1093/ehjci/jew056
- Kondo T, Uehara T. Immunoglobulin G4–related disease with fibroinflammatory lesions in the pleura, bile ducts and pericardium. Cmaj. 2016;188(13):972-. doi:10.1503/cmaj.160186
- González-Moreno J, Losada-López I, Gállego-Lezaun C, García-Gasalla M, Gómez Bellvert C, Ortego Centeno N. Serosal involvement in IgG4-related disease: report of two cases and review of the literature. Rheumatology international. 2016;36:1033-41. doi:10.1007/s00296-016-3501-8
- Terzic B, Spasic M, Djuric P, Vasiljevic V, Radjen S, Mijuskovic M. Retroperitoneal fibrosis and constrictive pericarditis-IgG4 related diseases: a case report. Experimental and Therapeutic Medicine. 2017;13(6):3603-6. doi:10.3892/etm.2017.4425
- Matsuda J, Takano H, Shimizu W. IgG4-related periarteritis in the coronary artery and subclinical pericarditis assessed the presence and monitoring of therapy response by PET and CT scan. Case Reports. 2018;2018:bcr-2018. doi:10.1136/bcr-2018-225172
- Steiner JM, Kataruka A, Burke C, Verrier E, Otto C. Immunoglobulin G4-Related Disease Manifesting As Constrictive Pericarditis. Journal of the American College of Cardiology. 2018;71(11S):A2405. doi:10.1016/S0735-1097(18)32946-2
- Weiss MA, Aubry MC, Pflederer BR. A case of IgG4-related Aortitis and pericarditis: diagnostic challenges and natural history. The American journal of case reports. 2018;19:1232. doi:10.12659/AJCR.910164
- Arao K, Mase T, Iwanami K, Nakai M, Sekiguchi H, Abe Y, et al. IgG4-related pericarditis in which oral corticosteroid therapy was effective. Internal Medicine. 2019;58(8):1119-22. doi:10.2169/internalmedicine.1403-18
- Gorecka MM, Armstrong R, Daly C. Unusual cause of pericardial effusion: IgG4-related disease. BMJ Case Reports. 2019 ;12(6):e230505. doi:10.1136/bcr-2019-230505
- Sly Z, Gorantla R. A Case Of Igg4-Related Constrictive Pericarditis. JACC. 2019, 73 (9_Supplement_1) 2616. doi:10.1016/S0735-1097(19)33222-X
- Tomoda Y, Kometani T, Kato S, Tanaka K. Immunoglobulin G4-related pericarditis. European Heart Journal. 2019;40(46):3803. doi:10.1093/eurheartj/ehz442
- Wang Y, Guo Y, Zhang R, Qi X, Zhong Y, Zhang W, et al. A Rare Constrictive Pericarditis Caused By Immunoglobulin G4-Related Disease. Journal of the American College of Cardiology. 2019;73(9S1):2707. doi:10.1016/S0735-1097(19)33313-3
- Yassi U, Iqbal F, Stevenson HL. IgG4-related sclerosing pericarditis in a young man with recurrent chest pain. The Annals of Thoracic Surgery. 2019;108(4):e261-3. doi:10.1016/j.athoracsur.2019.02.011
- Meier N, Lannan M, Mott R, Norton D. Suspected IgG4-Related Serositis: Tissue Isn't the Only Issue. InA34. Auto Immune Lung Disease: Case Reports 2020 (pp. A1367-A1367). American Thoracic Society. doi:10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A1367
- Yamamoto H, Ohta-Ogo K, Isogai J, Ishibashi-Ueda H, Nakamura Y. Epicardial Nodules as the Initial Manifestation of Immunoglobulin G4–Related Pericarditis. Case Reports. 2020;2(10):1505-9. doi:10.1016/j.jaccas.2020.05.092
- Yuriditsky E, Dwivedi A, Narula N, Axel L, Horowitz JM, Vaynblat M. Constrictive pericarditis caused by IgG4-related disease requiring pericardiectomy after partial response to corticosteroids. Case Reports. 2020;2(10):1558-63. doi:10.1016/j.jaccas.2020.06.038
- Corona-Rodarte E, Ramos-Coria D, Nuñez-Zuno JA, Plascencia-Félix JF, Delgado-de la Mora J, Galindo-Uribe J. An unusual case of purulent constrictive pericarditis with Hyper-IgG4-related histological findings on biopsy. Clinical Infection in Practice. 2021;10:100071. doi:10.1016/j.clinpr.2021.100071
- Doumen M, Vankelecom B, Westhovens R, Michiels S. Pericarditis as a manifestation of IgG4-related disease. Rheumatology International. 2022;42(7):1287-95. doi:10.1007/s00296-021-04946-9
- Fujita H, Matsumoto K, Miwa K, Hirata KI. “Pericardial flare” associated with immunoglobulin G4-related disease: A case report-rapid transition from cardiac tamponade to effusive constrictive pericarditis. Journal of Cardiology Cases. 2021;24(1):37-40. doi:10.1016/j.jccase.2020.12.007
- Majid M, Lak HM, Yaker ZS, Mazumder SH, Agrawal A, Syed A, et al. An Unusual Case of Transient Inflammatory Constrictive Pericarditis: IgG4 Disease. Circulation. 2022 Nov;146(Suppl_1):A10781. doi:10.1161/circ.146.suppl_1.10781
- Maltês S, Cabral M, Freitas P, Albuquerque C, Fernandes C, Moura D, et al. Immunoglobulin G4-related constrictive pericarditis and the importance of a thorough workup: a case report. BMC Cardiovascular Disorders. 2022;22(1):28. doi:10.1186/s12872-022-02468-1
- Ohman R, Napolitano JD, Watson AD. Curious Cachexia: Constrictive Pericarditis As The Manifestation Of Igg4-Related Disease. Journal of the American College of Cardiology. 2022;79(9_Supplement):2243. doi: 10.1016/S0735-1097(22)03234-X
- George B, Bain ER, Jarori U. A Rare Case Of Igg4-Related Disease Manifesting As Constrictive Pericarditis. Journal of the American College of Cardiology. 2023 Mar 7;81(8_Supplement):2653. doi:10.1016/S0735-1097(23)03097-8
- Kawanami Y, Kagawa Y, Endo A, Tanabe K. A case of IgG4-related pericarditis. European Heart Journal-Case Reports. 2023;7(9):ytad437. doi:10.1093/ehjcr/ytad437
- Lildar T, Li C, Solinas A, Shah M, Lopez MD. IGG4-RELATED AUTOIMMUNE PANCREATITIS RESULTS IN CARDIAC TAMPONADE. Journal of the American College of Cardiology. 2023;81(8_Supplement):2583. doi:10.1016/S0735-1097(23)03027-9
- Saad MA, Ahmed H, Elgohary R, El Gendy HI. IgG4 related pericardium and lung disease in pediatric patient complicated with fatal massive hemoptysis: a case report and review of literature. Pediatric Rheumatology. 2023;21(1):16. doi:10.1186/s12969-023-00799-7
- Sugawara H, Takahashi T, Kimura Y, Matsui A, Matsumoto T, Nishisato K, et al. IgG4‐Related Pericarditis Diagnosed by Accumulated Pericardial Effusion. Case Reports in Cardiology. 2023;2023(1):9223342. doi:10.1155/2023/9223342
- Wei Q, Qi H, Wei H, Wang X, Zhang H. IgG4-related disease with massive pericardial effusion diagnosed clinically using FDG-PETCT: a case report. Frontiers in Immunology. 2023;14:1285822. doi: 10.3389/fimmu.2023.1285822
- An SY, Sun BJ. Semiquantitative 18F-FDG PET/CT in monitoring glucocorticoid response of immunoglobulin G4-related effusive constrictive pericarditis: a case report. BMC Cardiovascular Disorders. 2024;24(1):122. doi:10.1186/s12872-024-03797-z
- Miura K, Takaoka H, Ota M, Irie R, Ota J, Noguchi Y, et al. Utility of Computed Tomography for the Initial Detection and Follow-up of Silent Coincidence of IgG4-Related Pericarditis, Periaortitis, and Coronary Arteritis. Circulation Reports. 2024;6(2):28-9. doi:10.1253/circrep.CR-23-0086
- Okabe T, Kawahata T, Koyanagi Y, Ito Y, Gibo Y, Okura T, et al. Rituximab and pericardiectomy with waffle procedure in constrictive pericarditis due to IgG4‐related disease: A case report. Clinical Case Reports. 2024;12(6):e8924. doi:10.1002/ccr3.8924
- Ozgur SS, Tagliaferri A, Aiken A, Desai B, Abboud R, Shamoon Y, et al. A rare case of immunoglobulin G4-related constrictive pericarditis diagnosed via multimodality imaging. Journal of Investigative Medicine High Impact Case Reports. 2024;12:23247096241248969. doi:10.1177/23247096241248969
- Shimada A, Yamamoto T, Dohi S, Endo D, Tabata M. Surgical treatment of chronic aortic dissection with liver dysfunction due to constrictive pericarditis caused by IgG4-related disease: a case report. European Heart Journal-Case Reports. 2024;8(9):ytae471. doi:10.1093/ehjcr/ytae471
- Son J, Lee SH. IgG4-related pericarditis. QJM: An International Journal of Medicine. 2024;117(4):300-1. doi:10.1093/qjmed/hcad284
- Thummala A, Ardehali A, Trang A, Bukiri H, Yang EH, Pelter M, et al. Constrictive Pericarditis As A Manifestation Of Igg4-Related Disease. Journal of the American College of Cardiology. 2024;83(13_Supplement):3722. doi:10.1016/S0735-1097(24)05712-7
- Ono K, Nomura T, Shoji K, Kato Y, Wada N. A very rare phenotype of immunoglobulin G4-related disease that was manifested as constrictive pericarditis: a case report. European Heart Journal-Case Reports. 2025;9(1):ytae689. doi:10.1093/ehjcr/ytae689
- Pons-Estel GJ, Ugarte-Gil MF, Alarcón GS. Epidemiology of systemic lupus erythematosus. Expert review of clinical immunology. 2017;13(8):799-814. doi:10.1080/1744666X.2017.1327352
- Mohammed SA, Karim DO, Fakhralddin SS, Bapir R, Hadi TS, Hussein DM, et al. Secondary renal amyloidosis due to primary Sjogren’s syndrome: a case report. Annals of Medicine and Surgery. 2023;85(6):3035-8. doi:10.1097/MS9.0000000000000721
- Scott IC, Whittle R, Bailey J, Twohig H, Hider SL, Mallen CD, et al. Rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis epidemiology in England from 2004 to 2020: an observational study using primary care electronic health record data. The Lancet Regional Health–Europe. 2022;23. doi:10.1016/j.lanepe.2022.100519
- Hama Hussein KF, Bapir R, Hiwa DS, Hama NH, Mohammed SA, Tahir SH, et al. Takayasu arteritis masquerading as brucellosis: a case report. Oxford Medical Case Reports. 2024;2024(12):omae147. doi:10.1093/omcr/omae147
- Mohammed SA, Kakamad FH, Tahir SH, Hassan MN, Hassan MN, Abdalla BA, et al. May-Thurner Syndrome as The Presenting Symptoms of Behcet’s Disease: A Rare Case Report. Barw Medical Journal. 2023;1(1):39-42. doi:10.58742/bmj.v1i1.17
- Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS. Multiple Sclerosis Journal. 2020;26(14):1816-21. doi:10.1177/1352458520970841
- Kakamad FH, Mohammed SA, Tahir SH, Jamal R, Baqi DH, Hussein DM, et al. Pulmonary Artery Aneurysm-Related Fatal Hemoptysis in Behcet's Disease: A Case Report with Literature Review. Barw Medical Journal. 2023;1(1):43-45. doi:10.58742/bmj.v1i1.18
- Bapir R, Hawrami TA, Aghaways I, Ali RM, Hiwa DS, Hussein DM, et al. A huge retroperitoneal ganglioneuroma in a middle-aged patient: report of a diagnostically challenging case with review of the literature. Oncology Letters. 2022;24(6):449. doi:10.3892/ol.2022.13569
- Zhou W, Murray T, Cartagena L, Lim H, Schaeffer DF, Slack GW, et al. IgG4-related disease as mimicker of malignancy. SN Comprehensive Clinical Medicine. 2021;3(9):1904-13. doi:10.1007/s42399-021-00957-6
- Ahmed HK, Hiwa DS, Tahir SH, Ali RM, Gharib DT, Asaad HR, et al. Crohn’s disease presenting with pleural effusion: a case report. Oxford Medical Case Reports. 2024;2024(10):omae113. doi:10.1093/omcr/omae113
- Wallace ZS, Naden RP, Chari S, Choi HK, Della-Torre E, Dicaire JF, et al. The 2019 American College of Rheumatology/European League against rheumatism classification criteria for IgG4-related disease. Annals of the rheumatic diseases. 2020;79(1):77-87. doi:10.1136/annrheumdis-2019-216561
- Pope JE, Choy EH. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. InSeminars in arthritis and rheumatism 2021 Feb 1 (Vol. 51, No. 1, pp. 219-229). WB Saunders. doi:10.1016/j.semarthrit.2020.11.005
- Hama Hussein KF, Abdullah HO, Ahmed SF, Qadir AN, Asaad HR, Fattah FH, et al. The clinical manifestations and treatment outcomes of Behçet's disease: A single‐center experience. Health Science Reports. 2024;7(7):e2238. doi:10.1002/hsr2.2238
- Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Critical reviews in clinical laboratory sciences. 2011;48(4):155-70. doi:10.3109/10408363.2011.599831
- Abdullah HO, Braim SA, Rasool MA, Abdalla DM, Hamad DQ, Ahmed DK, et al. Role of Inflammatory Markers in Severity, ICU Admission, and Mortality in COVID-19: A Systematic Review and Meta-analysis of 79,934 Patients.Barw Medical Journal.2024;2(3):65-83. doi:10.58742/bmj.v2i2.96
- Lilly LS. Treatment of acute and recurrent idiopathic pericarditis. Circulation. 2013;127(16):1723-6. doi:10.1161/CIRCULATIONAHA.111.066365
- Imazio M, Gaita F. Diagnosis and treatment of pericarditis. Heart. 2015;101(14):1159-68. doi:10.1136/heartjnl-2014-306362

This work is licensed under a Creative Commons Attribution 4.0 International License.

