Renal Ewing Sarcoma: A Case Report and Literature Review
Abstract
Introduction
Primary renal Ewing sarcoma is an extremely rare and aggressive tumor, representing less than 1% of all renal tumors. This case report contributes valuable insights into the challenges of diagnosing and managing this rare malignancy, particularly when it presents with atypical symptoms.
Case presentation
A 30-year-old female presented with intermittent grassy green-colored urine, later turning into red, with a headache and no abdominal pain. Clinical examination revealed elevated blood pressure. Imaging studies, including ultrasound and computed tomography scans, identified a large, heterogeneous mass in the left kidney with invasion into the renal vein and lymph nodes, leading to a staging of T3N1M0. The patient underwent a left radical nephrectomy, with pathology confirming a Grade 2 primary Ewing sarcoma / primitive neuroectodermal tumor of the kidney. Despite aggressive treatment with adjuvant chemotherapy, stable metastatic deposits persisted, indicating ongoing active disease.
Literature review
Ewing sarcoma typically occurs in bones but can occasionally arise in solid organs such as the kidney. Most patients present with non-specific symptoms, and the disease often remains undiagnosed until it has metastasized. Current treatment involves multimodal therapy, including surgery and chemotherapy, but prognosis remains poor, especially in cases with metastasis.
Conclusion
This case underscores the complexity of diagnosing and treating primary renal Ewing sarcoma. Persistent metastasis despite treatment highlights the need for vigilant monitoring. Further genetic profiling could enhance understanding and management of this rare condition.
Introduction
Sarcomas are a diverse group of malignant tumors originating from mesenchymal tissues, and they can arise in virtually any part of the body, often posing diagnostic and therapeutic challenges due to their rarity and varied presentation. Recent reports have highlighted unusual locations and rare co-occurrences of sarcomas, emphasizing the need for heightened clinical suspicion when encountering atypical masses [1,2].
Ewing sarcoma (EWS) is a highly aggressive tumor typically found in the bones of children and young adults. However, it can occasionally originate from solid organs that contain neuroendocrine cells, such as the kidney, lungs, heart, bladder, small intestine and parotid glands, with approximately 6% of cases being extraosseous [3-5]. Ewing sarcoma is classified as a small round cell sarcoma characterized by gene fusions between a member of the Ewing Sarcoma Breakpoint Region 1 (EWSR1) gene family and a member of the E26 transformation-specific sequence (ETS) family of transcription factors [6]. Primary EWS of the kidney is an extremely rare tumor, comprising less than 1% of all renal tumors, with fewer than 200 cases reported globally. However, the precise number of cases is challenging to determine, as these tumors are not always accurately diagnosed. [3,7]. It is a highly aggressive tumor that primarily affects young individuals, with a particular prevalence among males [8]. Patients typically present with an abdominal mass or renal symptoms such as abdominal pain and hematuria. Due to its hidden intra-abdominal location, the tumor often grows to a significant size before being detected [9]. Primary kidney EWS metastasizes to the bone, lungs, and lymph nodes. Its clinical presentation is not specific, and it can resemble other renal tumors in histologic appearance [10]. In this case, a 30-year-old female presented with primary renal EWS, exhibiting unusual symptoms such as grassy green to red urine. The references’ eligibility has been verified [11]. The report is structured in accordance with CaReL guidelines and includes a recent review of the literature [12].
Case Presentation
Patient information
A 30-year-old female presented with a chief complaint of intermittent grassy green-colored urine, which later became red. She also experienced headaches but reported no fever, vomiting, or abdominal pain. Her past medical history was unremarkable, though she had undergone the removal of an ovarian cyst on the left side just one month prior to her diagnosis.
Clinical findings
On clinical examination, there was no abdominal tenderness or other systemic abnormalities. Her vital signs were normal except for elevated blood pressure, recorded at 120/100 mmHg.
Diagnostic approach
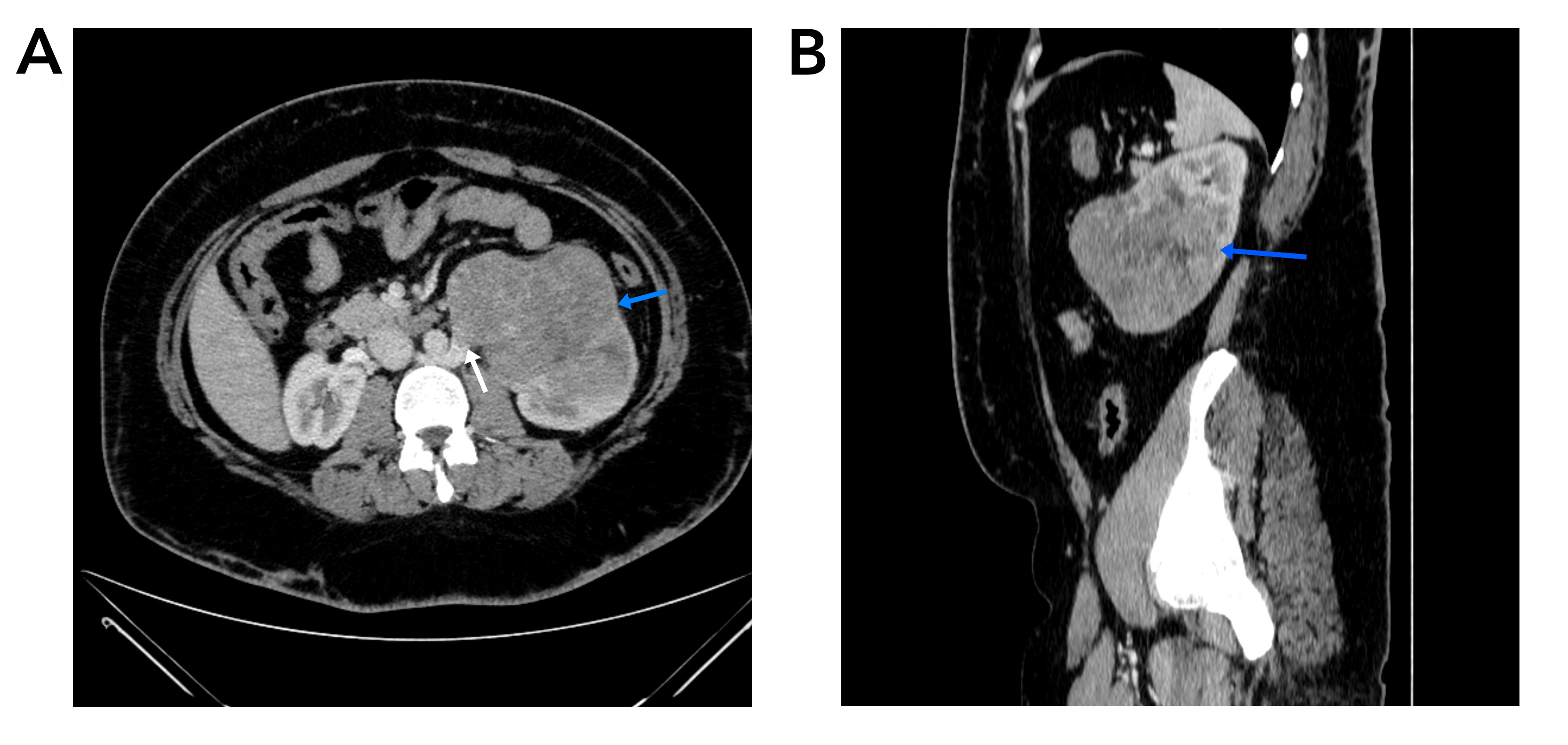
Diagnostic investigations included abdominal ultrasonography, which revealed an enlarged and irregularly shaped left kidney with mild pelvicalyceal system dilation. A large heterogeneous iso to hyper-echoic mass measuring 11x10x8 cm was identified, extending from the mid to lower pole of the kidney and displaying internal vascularity. A subsequent computed tomography (CT) scan of the abdomen confirmed the presence of a large heterogeneous solid lesion at the lower pole of the left kidney, measuring approximately 12x10x8.5 cm. The mass showed heterogeneous enhancement after contrast administration and extended into the renal pelvis and lower calyx, with associated mild dilation. The scan also revealed invasion of the retroaortic right renal vein and two enlarged locoregional lymph nodes, the largest measuring 6 mm in short axis. These findings were indicative of a malignant renal tumor, staged as T3N1M0 (Figure 1).
Therapeutic interventions
The patient underwent a left radical nephrectomy. Histopathological examination confirmed the diagnosis of a primary EWS /primary neuroectodermal tumor of the kidney, classified as Grade 2, with a mitotic activity of 6-8 per 10 high-power fields (Figure 2). The tumor, which measured 10 cm at its greatest dimension, was located in the lower pole of the left kidney and was unifocal.
There was no evidence of tumor necrosis, lymphovascular invasion, or positive surgical margins, though one out of eight examined lymph nodes tested positive for metastasis. The final pathological stage was pT1pN1M0. Immunohistochemistry showed that the tumor cells were positive for CD99, while other markers such as AE1/AE3, CD45, desmin, S100, synaptophysin, CD31, and WT1 were negative. The patient subsequently received adjuvant chemotherapy with the VAC-IE regimen (vincristine, doxorubicin, cyclophosphamide-ifosfamide, etoposide).
![Figure 2) A-B) The tumor is composed of sheets of small, round, blue cells. C-D) The tumor cells have scant lightly eosinophilic to clear cytoplasm with round, hyperchromatic nuclei. [Hematoxylin and eosin; original magnification x 40 (A), x 100 (B), x 400 (C and D)].](https://www.barwmedical.com/public/site/images/12abdalla12/mceu-23520417231750063849007.jpg)
Follow-up
One month after the operation, a magnetic resonance imaging (MRI) of the abdomen revealed a 50x20 mm elongated, well-defined area of bright signal on T1 and T2 imaging, with no diffusion-weighted imaging restriction or enhancement, overlapping the left psoas muscle. This finding was suggestive of a postoperative hematoma. A high-resolution CT scan of the chest was normal. Three months later, a fluorodeoxyglucose positron emission tomography (FDG-PET) scan showed multifocal areas of FDG avidity extending from the lateral aspect of the crus of the left hemidiaphragm and left psoas muscle, with a maximum standardized uptake value of 6.53. The largest hypermetabolic lesion measured 1.2x1.1 cm. A laparoscopic biopsy of a nodule on the small bowel serosa and psoas muscle showed benign findings with no evidence of malignancy.
Over the course of the follow-up, two additional FDG-PET scans were performed, three months apart. The latest scan revealed a stable metastatic deposit in the left nephrectomy bed along the left psoas muscle with a slight decrease in FDG uptake (less than 30%). The disease was considered interval stable, and although radiation treatment was recommended, it was ultimately deemed unnecessary. The patient continued to be monitored, with two additional abdominal MRIs conducted four months apart. The most recent MRI showed a non-enhancing lesion measuring 67x29x20 mm, consistent with previous findings.
Discussion
Most cases of EWS develop in the bones, with the lower extremities and pelvis being the most common sites of occurrence. However, it is extremely rare for EWS to appear as a primary tumor in the kidney. The cells responsible for EWS are believed to originate from neural crest cells or mesenchymal stem cells [9, 13]. Approximately 66% of patients have distant metastasis at the time of diagnosis, with the lungs being the most common site, followed by the liver and bones [4]. The median age for this condition is 27 years, with a slight predominance in males. The symptoms are non-specific and include flank pain in 84% of cases, a palpable mass in 60%, and hematuria in 38% [6]. The review of 19 cases of EWS of the kidney in this study (Table 1) revealed a mean age of 24.5 years, with females accounting for 57.9% of the cases. The most common symptoms were flank pain (47.36%), followed by hematuria (42.1%) and abdominal pain (36.8%). The average tumor size was 12.27 cm. In contrast, the current case involved a 30-year-old female who presented with intermittent grassy green-colored urine and a headache, but no abdominal pain.
Diagnosis can be assisted using radiological techniques such as MRI and CT scans, in determining the tumor's size, location, and the extent of both local and distant metastasis [9]. The definitive diagnosis of renal EWS relies on pathological, immunohistochemical, and molecular testing. Microscopically, the majority of cases consist of uniform small round cells with round nuclei, finely stippled chromatin, subtle nucleoli, scant clear or eosinophilic cytoplasm, and indistinct cytoplasmic membranes [6]. The protein K2.2 plays a role in regulating gene expression within the neuroendocrine and glial differentiation pathways, and NKX2.2 serves as a specific marker for identifying the EWS/FLI1 fusion protein, exhibiting a high sensitivity of 93% and a specificity of 89% [6]. In the current case, abdominal ultrasonography revealed an enlarged, irregularly shaped left kidney with a large mass sized 10 cm that was confirmed by CT scan, which was smaller than ten and larger than seven of the cases in this literature review (Table 1).
| Author and Year | No. of Cases |
Age Gender |
Clinical Presentation |
Medical & Surgical History | Diagnostic Method |
Size and Site of Tumor |
Treatment | Outcome | |
| Conservative | Surgical | ||||||||
|
Aithal et al., 2024[4] |
1 |
40 F |
Painless hematuria |
None reported |
MRI, CECT, Histo, IHC |
7x5.5x5 cm, Lower pole, LK |
Chemotherapy |
Nephrectomy |
N/A |
|
Alahmadi et al., 2020[8] |
1 |
16 M |
Hematuria, flank pain |
Schizophrenia |
CECT, Histo, IHC |
19 × 15 × 12 cm, RK |
Chemotherapy |
Nephrectomy |
Remission |
|
Bradford et al., 2020[14] |
Case 1 |
16 M |
Abdominal pain |
N/A |
IHC |
RK |
VDC/IE; |
Partial Nephrectomy |
Died |
|
Case 2 |
11 M |
Flank and testicle pain, fever |
N/A |
Ultrasound, CT, IHC |
28×17×7.3 cm, LK |
VDC/IE; auto SCT; everolimus, Chemotherapy |
Nephrectomy |
Alive with disease |
|
|
Case 3 |
18 F |
Hematuria, right flank pain |
N/A |
PET, CT |
6.8×6.2×6.0 cm, RK |
VDC/IE |
Radical nephrectomy, IVC tumor removal |
Remission |
|
|
Case 4 |
17 F |
N/A |
N/A |
PET |
N/A |
Chemotherapy, VDC/IE |
Tumor resection |
Remission |
|
|
Case 5 |
16 F |
N/A |
N/A |
N/A |
25×18×19 cm, LK |
VDC/IE |
Gross total resection |
Remission |
|
|
Case 6 |
13 F |
Abdominal pain, spinal cord compression |
N/A |
MRI, PET, Histo |
6.8×14×13 cm, LK |
VDC/IE |
Nephrectomy, following neoadjuvant chemotherapy |
Died |
|
|
Case 7 |
15 F |
Abdominal pain, ataxia, elevated urate and creatinine |
N/A |
N/A |
21 cm, LK |
Chemotherapy, VDC/IE switched to VDC/CE for ifosfamide nephrotoxicity |
Radical nephrectomy, partial ureterectomy, IVC thrombectomy |
Died |
|
|
Bray et al., 2023[13] |
1 |
31 F |
Macroscopic hematuria, flank pain |
unremarkable |
CT, MRI, Ultrasound |
11.6 cm, RK |
VDC/IE Chemotherapy |
laparoscopic radical Nephrectomy |
Remission |
|
Cheng et al., 2020[15] |
1 |
31 F |
Hematuria, flank pain, palpable mass |
unremarkable |
CT, 3D imaging, PET, Histo, IHC |
18×14.5×14 cm, LK |
Chemotherapy |
Nephrectomy |
Metastasis |
|
El Mohtarim et al., 2024[6] |
1 |
14 F |
Flank swelling, abdominal pain weight loss |
2 mo of abdominal pain |
MRI, CT, Histo, IHC |
20×16×14 cm, RK |
Chemotherapy, VDC/IE |
Nephrectomy |
Remission |
|
Ilhan et al., 2023[7] |
1 |
54 M |
Flank pain, hematuria |
Smoker, Alcohol consumption |
Ultrasound, CT, Histo, IHC |
7.4×6.3 cm, RK |
Chemotherapy (VAC-IE) |
Nephrectomy |
Remission |
|
Khudair et al., 2024[9] |
1 |
38 F |
Abdominal pain, flank Pain |
Constipation, vomiting, morbid obesity |
Ultrasound, CT, Histo |
25×18×18 cm, RK |
Chemotherapy |
N/A |
Died |
| Patra et al., 2022[10] |
Case 1 |
33 F |
Abdominal pain |
N/A |
CECT, biopsy, Histo, IHC |
7.4 cm, RK |
Chemotherapy |
Nephrectomy |
Died |
|
Case 2 |
35 M |
Palpable lump |
N/A |
CECT, biopsy, Histo, IHC |
4.5 cm, LK |
Chemotherapy |
Nephrectomy |
Remission |
|
|
Case 3 |
19 M |
Palpable lump, hematuria |
N/A |
CECT, biopsy, Histo, IHC |
19 cm, RK |
NACT |
Nephrectomy |
Alive with disease |
|
|
Case 4 |
28 M |
Abdominal pain |
N/A |
CECT, biopsy, Histo, IHC |
6.4 cm, LK |
NACT |
Nephrectomy |
Alive with disease |
|
|
Sardana et al., 2021[3] |
1 |
49 M |
Gross hematuria, flank pain |
GERD, asthma, hyperlipidemia |
MRI, CT, Histo, IHC |
7.0×6.2×5.8 cm, RK |
Chemotherapy, Radiotherapy |
Nephrectomy |
Remission |
| F: female M: male N/A: not applicable mo: months MRI: magnetic resonance imaging CECT: contrast enhanced computed tomography Histo: histopathology IHC: immunohistochemistry PET: positron emission tomography LK: left kidney RK: right kidney NACT: Neoadjuvant chemotherapy IVC: inferior vena cava VDC/IE: vincristine, doxorubicin, and cyclophosphamide/ ifosfamide, etoposide y: year | |||||||||
To establish a definitive diagnosis, the patient underwent a left radical nephrectomy. Similarly, in the cases by Patra et al., histopathological examination confirmed the tumor as a Grade 2 primary EWS /PNET of the kidney [10]. Despite its aggressive nature, there was no evidence of distant metastasis in the present patient, and she received adjuvant chemotherapy, which was in line with the cases by Ilhan et al. and Bradford et al. [7,14]. In contrast, Cheng et al. reported metastatic spread to multiple sites, including lymph nodes, adrenal glands, and lungs, requiring a switch to apatinib, with the patient surviving 18 months postoperatively [15].
The most common treatment approach currently involves multimodal therapy, which includes surgery and adjuvant chemotherapy for localized EWS of the kidney [7]. The current patient underwent a left radical nephrectomy to remove the primary tumor. The surgery was successful in excising the tumor. The pathological examination revealed no tumor necrosis, lymphovascular invasion, or positive surgical margins, but one out of eight examined lymph nodes tested positive for metastasis, leading to a final pathological stage of pT1pN1M0.
Immunohistochemical analysis confirmed the diagnosis of EWS/PNET, with the tumor cells showing positivity for CD99, a marker commonly associated with EWS. Other markers, including AE1/AE3, CD45, desmin, S100, synaptophysin, CD31, and WT1, showed negatively, and Ki67 showed a proliferation index of 20%. In contrast, Cheng et al. reported positive results for AE1/AE3, CD99, CD56, and synaptophysin [15]. Following surgery, the patient received adjuvant chemotherapy using the VAC-IE regimen similar to Ilhan et al., which includes vincristine, doxorubicin, cyclophosphamide (VAC), ifosfamide, and etoposide (IE). This multimodal chemotherapy approach was implemented to target any remaining microscopic disease and to lower the risk of tumor recurrence [7].
The current case did not achieve full remission due to stable metastatic deposits, indicating active cancer. In contrast, the patients in the studies of both Ilhan et al. and Bray et al. remained in remission with no disease progression, reflecting more
favorable outcomes [7,13]. The prognosis of EWS depends on various factors, including tumor location and size, presence of metastatic disease, and treatment plans. However, the impact of age on disease outcomes remains uncertain [3]. In the present report, while the disease is stable, the persistence of metastasis suggests ongoing risk, requiring continuous monitoring. Detailed genetic profiling of the tumor was not included, which could have provided deeper insights into the disease's molecular mechanisms and influenced treatment strategies.
Conclusion
The current case highlights that EWS of the kidney can present with grassy green-colored urine and hematuria. Radical nephrectomy with the VAC-IE regimen may result in good outcomes with continuous monitoring required in cases of metastatic deposits.
Declarations
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethical approval: Not applicable
Patient consent (participation and publication): Written informed consent was obtained from the patient for publication.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: RMA and SMA were significant contributors to the conception of the study and the literature search for related studies. MAG, DSH and JSA were involved in the literature review, study design, and manuscript writing. ZNH, HAY, AMA, SSF, BSS and AKG were involved in the literature review, the study's design, and the critical revision of the manuscript, and they participated in data collection. SHT was the radiologist who performed the assessment of the case. RMA was the pathologist who performed the diagnostic of the case. RMA and MAG confirm the authenticity of all the raw data. All authors approved the final version of the manuscript.
Use of AI: ChatGPT-3.5 was used to assist in language editing and improving the clarity of the manuscript. All content was reviewed and verified by the authors. Authors are fully responsible for the entire content of their manuscript.
Data availability statement: Note applicable.
References
- Fatah Mariwan L, Salh Abdulwahid M, Abdullah Ari M, Kakamad Fahmi H, Mustafa Shevan M, Kakamad Suhaib H. Adult embryonal rhabdomyosarcoma in the nasal cavity; a case report with a review of the literature. Annals of Medicine & Surgery. 2022; 75. | doi:10.1016/j.amsu.2022.103424
- Bapir R., Shabur B., Aghaways I., Tahir S.H., Abdullah H.O., Ahmed R.S, et al. (2023). Synchronous Kaposi sarcoma and renal cell carcinoma in an elderly male patient (a very uncommon reported entity): A case report. Medicine International, 3, 16. doi:10.3892/mi.2023.76
- Sardana R, Parwani A V., Shabsigh A, Sheldon J. An unusual case of renal EWS: A case report and review of literature. Human Pathology: Case Reports. 2021;25. doi:10.1016/j.ehpc.2021.200537
- Aithal V, Sajitha K, Mathias M. EWS of Kidney: A Rare Entity. Journal of Health and Allied Sciences NU. 2024; doi:10.1055/s-0044-1786993
- Ali R.M., Seerwan K., Ali S.M., Abdullah A.M., Hawrami O., Hussein D.M, et al. Primary pancreatic synovial sarcoma: Report of a rare case and review of the literature. Medicine International. 2023; 3, 22. doi:10.3892/mi.2023.82
- El Mohtarim R, Yassine Aaboudech T, Sassi S, Rguieg N, Cherraqi A, Dokal ID, et al. Primary EWS of the kidney: a rare entity with diagnostic challenges. J Surg Case Rep. 2024;2024(6): rjae390. doi:10.1093/jscr/rjae390
- Ilhan yusuf. Primary EWS of the Kidney in a 54-Year-Old Male: A Rare Case Report. Eurasian Journal of Medical Advances. 2023; doi:10.14744/ejma.2023.46855
- AlAhmadi HH, AlEssa A, Ahmed A, Al hamad MA, Fadaak K, El Darawany HM, et al. Primary EWS /primitive neuroectodermal tumor of the kidney. J Pediatr Surg Case Rep. 2020;61. doi:10.1016/j.epsc.2020.101608
- Khudair AD, Khudair AD, Al-Rawahia T, Marshall RA, Albenjasim K, Roohi M, et al. Unveiling the Uncommon: A Case of Metastatic EWS of the Kidney. Cureus. 2024; doi:10.7759/cureus.52970
- Patra S, Trivedi P. Primary EWS of the kidney: A series of four cases. Malaysian Journal of Pathology. 2022;44(1). 93-99. doi:N/A
- Kakamad FH, Abdalla BA, Abdullah HO, Omar SS, Mohammed SH, Ahmed SM, et al. Lists of predatory journals and publishers: a review for future refinement. European Science Editing. 2024;50:e118119. doi:10.3897/ese.2024.e118119
- Sakshi Prasad, Mahmoud Nassar, Ahmed Y. Azzam, Francisco García-Muro-San José, Mahnaz Jamee, Rim Kasem Ali Sliman, et al. CaReL Guideline: A Consensus-Based Guideline on Case Reports and Literature Review (CaReL). Barw Medical Journal. 2024; 2(2):13-19. doi:10.58742/bmj.v2i2.89
- Bray G, Nesbitt A, Maré A, Willis T, Tracey C. Primary EWS of the kidney presenting as haematuria in a pregnant woman – A case report highlighting the diagnostic and therapeutic dilemmas of the condition in pregnancy. Urol Case Rep. 2023;46. doi:10.1016/j.eucr.2022.102308
- Bradford K, Nobori A, Johnson B, Allen-Rhoades W, Naik-Mathuria B, Panosyan EH, et al. Primary Renal EWS in Children and Young Adults. J Pediatr Hematol Oncol. 2020;42(8). doi:10.1097/MPH.0000000000001804
- Cheng L, Xu Y, Song H, Huang H, Zhuo D. A rare entity of Primary EWS in kidney. BMC Surg. 2020;20(1). doi:10.1186/s12893-020-00948-9

This work is licensed under a Creative Commons Attribution 4.0 International License.

