Giant Sacrococcygeal Teratoma in Infant: Systematic Review
Abstract
Introduction
Sacrococcygeal teratoma (SCT) is a rare embryonal tumor that occurs in the sacrococcygeal region, with an incidence of about 1 in 35,000 to 40,000 live births. This study presents a systematic review of giant SCT greater than 10 cm.
Methods
A systematic review of published studies regarding giant SCT in infants was conducted. The studies included met the following criteria: 1) the teratoma was situated in the sacrococcygeal region; 2) all case reports involved infants with a teratoma larger than 10 cm, and 3) the size of the teratoma was verified to exceed 10 cm through diagnostic methods.
Results
The current study included 31 studies that met the inclusion criteria. The studies included patients aged 6.1 to 9.3 months, with a median age of 7.6 months, predominantly female (72.7%). Diagnoses were primarily made in the first and second trimesters (39%) or after birth (33.3%), with cesarean delivery being the most common method (66.7%). Tumors weighed between 1.5 and 5 kg, with an average diameter of 15.6 cm. Surgical resection was performed in 93.9% of cases. The most common complication was respiratory failure (30.3%), and histopathology revealed that 39.4% of tumors were immature teratomas, while 33.3% were mature teratomas. The overall survival rate was 66.7%, with 18.2% of survivors experiencing tumor recurrence. Most complications occurred in the second trimester; however, no significant associations were found concerning the timing of diagnosis. Additionally, tumor size did not significantly impact outcomes.
Conclusion
Routine ultrasound and MRI are essential for the antenatal diagnosis of SCT. Due to the high risk of morbidity with larger tumors, cesarean delivery is advised for tumors over 10 cm. Coccygectomy is the most effective approach to prevent recurrence, highlighting the importance of timely surgical intervention and ongoing follow-up.
Introduction
Sacrococcygeal teratoma (SCT) is an uncommon embryonal tumor form in the sacrococcygeal region. It affects around one among every 35,000 to 40,000 live births [1]. The condition is significantly more common in females, with a female-to-male ratio of 3:1 to 4:1. Teratomas consist of tissues originating from all three germ layers: ectoderm, endoderm, and mesoderm [2,3]. Although their exact embryonic origin remains uncertain, SCTs are believed to arise early in gestation from totipotent cells in Hensen’s node, a remnant of the primitive streak in the coccygeal region [2]. SCTs can vary significantly in size, with some growing large enough to cause noticeable anatomical changes, such as the anterior displacement of the anus and resulting in clinical symptoms like anal displacement, tightening of the anal canal, and constipation, often due to tumor compression of the bladder or rectum [1,3]. Obstetric ultrasound during the second trimester is a crucial tool for making antenatal diagnoses of tumors, helping to prevent perinatal and neonatal complications such as fetal hydrops, tumor rupture, placentomegaly, and high-output cardiac failure, all of which are linked to a higher risk of mortality [4,5]. In 1973, according to the American Academy of Pediatrics Surgical Section (AAPSS), Altman classified SCTs into four types based on the tumor's intrapelvic and intra-abdominal extension and external components. SCTs observed at birth are typically classified as Altman Type I or II, with Type III being rare, while Type IV is usually identified later in life [2]. The management of SCT involves surgical excision, and the likelihood of recurrence after complete removal is minimal [5], leading to a favorable prognosis. However, long-term follow-up is essential to monitor for recurrence [3].
While there have been reports of large SCTs, to the best of our knowledge, based on an extensive literature review and previous case reports, no systematic reviews specifically address SCTs greater than 10 cm in infants. All included references were confirmed for eligibility [6].
Methods
Study design
The present systematic review adhered to the preferred reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data sources and search strategy
A comprehensive review of all published studies on SCT was conducted by searching databases such as Google Scholar, PubMed/MEDLINE, Cochrane Library, ScienceDirect, CINAHL, Web of Science, and EMBASE. The search used the following keywords: (sacrococcygeal) AND (teratoma OR tumor) in combination with (antenatal OR neonate OR infancy OR infant OR child OR children OR newborn OR pediatric).
Eligibility criteria
The non-English studies and those unrelated to humans were excluded before or during the initial screening. Studies on SCT were included if they met the following criteria: 1) the teratoma was located in the sacrococcygeal region; 2) all case reports involved infants with a teratoma larger than 10 cm, and 3) the teratoma size was confirmed to be over 10 cm through diagnostic methods. Studies published in predatory journals (inappropriately peer-reviewed) and those not meeting the inclusion criteria were excluded.
Study selection and data extraction
The titles and abstracts of the identified studies were initially screened. This was followed by a comprehensive review of the full text to assess eligibility. Data points collected from the included studies included the year and country of the study, patient age and gender, age at delivery, trimester of detection, postnatal diagnosis, mode of delivery, tumor size and weight, imaging technique, complications, and recurrence rates.
Statistical analysis
The data were first utilized in a qualitative synthesis, followed by quantitative re-analysis using the Chi-square test and Fisher's exact test for categorical variables and the independent sample t-test for numerical data, conducted with SPSS software version 25.0. A significance level of 0.05 was not established.
Results
Study selection and characteristics
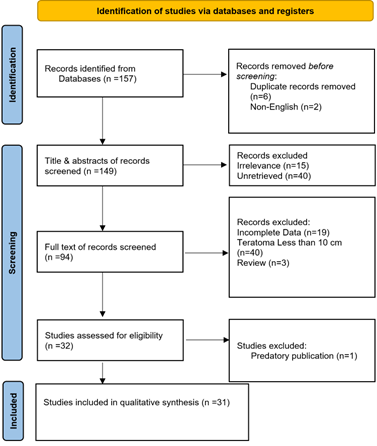
A systematic search initially identified 157 articles. Before screening, eight were excluded due to duplication and non-English language. During the initial title and abstract screening, 55 studies were excluded as they did not meet the inclusion criteria. Subsequently, 94 studies underwent full-text screening, and 32 were further assessed for eligibility. Ultimately, 31 studies [1-4, 7-33] were compatible with the inclusion criteria and included in the final analysis (Figure 1). All the studies included were case reports (Table 1).
|
Author [reference] |
Year |
Country |
Gender |
Age of delivery (days) |
Diagnosis time |
Mode of delivery |
Weight (kg) |
Size of tumor (cm) |
Altman classification |
Complication |
Histopathology |
Survival |
Recurrence |
|
Chamberlin et al. [7] |
N/A |
USA |
N/A |
224 |
1st trimester |
CS |
3.7 |
13.9 |
N/A |
Near cardiac arrest & hypoxia |
N/A |
Yes |
N/A |
|
Sabir et al. [1] |
2023 |
Iraq |
F |
266 |
2nd trimester |
CS |
5.0 |
17.5 |
1 |
None |
Immature teratoma |
Yes |
No |
|
Dey et al. [8] |
2023 |
USA |
F |
206 |
Prenatal (Uknown trimester) |
CS |
2.4 |
11.6 |
3 |
Poor respiratory effort |
Immature teratoma |
Yes |
No |
|
Abou-Bekr et al. [4] |
2022 |
Algeria |
F |
N/A |
Postnatal |
CS |
N/A |
10 |
1 |
None |
Mature teratoma |
Yes |
N/A |
|
Koc et al. [9] |
2022 |
Turkey |
F |
209 |
N/A |
N/A |
2.2 |
10 |
1 |
None |
Teratoma (unknown type) |
Yes |
N/A |
|
Meshram et al. [2] |
2021 |
India |
M |
259 |
2nd trimester |
CS |
2.2 |
24.5 |
2 |
Polyhydramnios |
Mature teratoma |
No |
N/A |
|
Zlatan et al. [10] |
2021 |
Bosnia and Herzegovina |
M |
238 |
2nd trimester |
CS |
2.3 |
15.5 |
2 |
Cardiac arrest |
Immature teratoma |
Yes |
N/A |
|
Guitart et al. [11] |
2020 |
Spain |
N/A |
245 |
2nd trimester |
CS |
4.0 |
18 |
1 |
Premature rupture of the membranes & abnormal cardiotocography |
Immature teratoma |
Yes |
N/A |
|
Savitri et al. [12] |
2019 |
Indonesia |
F |
266 |
2nd trimester |
CS |
4.2 |
11.2 |
1 |
Resuscitation needed |
Teratoma (unknown type) |
Yes |
No |
|
Singhal et al. [13] |
2018 |
India |
F |
196 |
Postnatal |
NVD |
3.6 |
16 |
N/A |
Infection |
Mature teratoma |
N/A |
N/A |
|
Konoplitskyi et.al [14] |
2018 |
Ukraine |
F |
280 |
3rd trimester |
CS |
4.3 |
14.9 |
1 |
N/A |
Immature teratoma |
Yes |
Yes |
|
Sop Lee et al. [15] |
2017 |
Korea |
M |
199 |
2nd trimester |
CS |
2.9 |
12.3 |
3 |
Poor respiratory distress |
Immature teratoma |
Yes |
No |
|
Bechtel et.al [16] |
2014 |
USA |
N/A |
226 |
Prenatal (Uknown trimester) |
CS |
3.7 |
13.9 |
2 |
Lower limb flaccid paralysis, scoliosis |
Immature teratoma |
Yes |
Yes |
|
Mondal et al. [17] |
2014 |
India |
F |
245 |
Postnatal |
NVD |
3.5 |
16.7 |
1 |
Bleeding |
N/A |
No |
N/A |
|
Mbumba et al. [18] |
2010 |
France |
F |
266 |
1st trimester |
CS |
N/A |
16 |
N/A |
None |
Mature teratoma |
N/A |
N/A |
|
Roka et al. [3] |
2010 |
Nepal |
F |
N/A |
Postnatal |
N/A |
N/A |
22.4 |
N/A |
None |
Mature and yolk sac components (Schiller-Duval bodies) |
Yes |
N/A |
|
Lahdes-Vasama et al. [19] |
2010 |
Finland |
F |
210 |
2nd trimester |
CS |
3.4 |
16.9 |
2 |
Bleeding |
Immature teratoma with a malignant component in a small area |
Yes |
No |
|
Abraham et al. [20] |
2010 |
USA |
F |
238 |
2nd trimester |
CS |
N/A |
18.9 |
3 |
Premature rupture of membranes & poor respiratory effort |
N/A |
Yes |
N/A |
|
Den Otter et al. [21] |
2007 |
Netherlands |
F |
192 |
2nd trimester |
CS |
2.2 |
11 |
2 |
Respiratory failure |
Mixed teratoma |
Yes |
Yes |
|
Howman-Giles et al. [22] |
2007 |
Australia |
F |
N/A |
Postnatal |
N/A |
N/A |
16 |
N/A |
Left iliac lymph node enlargement, colostomy needed |
Immature teratoma |
No |
Yes |
|
Hosono et al. [23] |
2004 |
Japan |
F |
252 |
3rd trimester |
CS |
4.0 |
13.1 |
1 |
Poor respiratory efforts |
Immature teratoma |
Yes |
No |
|
Ribeiro et al. [24] |
1999 |
France |
F |
266 |
Postnatal |
CS |
2.7 |
11.4 |
1 |
None |
Mature teratoma |
Yes |
No |
|
Jona et al. [25] |
1999 |
USA |
F |
189 |
Prenatal (Uknown trimester) |
CS |
1.7 |
26 |
N/A |
N/A |
Teratoma (unknown type) |
No |
N/A |
|
Johnston [26] |
1998 |
USA |
N/A |
245 |
N/A |
N/A |
3.9 |
12.2 |
N/A |
N/A |
Mature elements, except for immature neuroectodermal tissue. |
Yes |
N/A |
|
Robertson et al. [27] |
1995 |
USA |
F |
185 |
2nd trimester |
CS |
1.8 |
N/A |
2 |
Severe respiratory distress syndrome |
Mature teratoma |
Yes |
No |
|
Lnoue et al. [28] |
1994 |
Japan |
M |
210 |
2nd trimester |
CS |
3.8 |
10 |
N/A |
Bleeding and circulatory failure |
Immature teratoma |
Yes |
N/A |
|
Nakayama et al. [29] |
1991 |
USA |
F |
231 |
3rd trimester |
CS |
4.3 |
13.9 |
2 |
Respiratory insufficiency & renal failure |
Immature teratoma |
N/A |
N/A |
|
1991 |
USA |
F |
210 |
Postnatal |
CS |
3.0 |
15 |
3 |
Respiratory distress |
Immature teratoma |
N/A |
N/A |
|
|
Worsham et al. [30] |
1975 |
USA |
F |
189 |
Postnatal |
NVD |
1.9 |
13.1 |
N/A |
No respiratory efforts |
N/A |
No |
N/A |
|
Williams et al. [31] |
1970 |
Nigeria |
F |
266 |
Postnatal |
N/A |
N/A |
30 |
N/A |
Metastasis |
Teratoma (unknown type) |
Yes |
Yes |
|
Schiffer et al. [32] |
1956 |
USA |
F |
196 |
3rd trimester |
NVD |
1.5 |
20 |
N/A |
Difficult birth |
N/A |
N/A |
N/A |
|
1956 |
USA |
N/A |
N/A |
Postnatal |
NVD |
N/A |
15 |
N/A |
Difficult birth |
N/A |
N/A |
N/A |
|
|
Walker et al. [33] |
1950 |
USA |
F |
224 |
Postnatal |
NVD |
2.7 |
12.5 |
N/A |
N/A |
Teratoma (unknown type) |
Yes |
N/A |
| N/A: non-available, F: female, M: male, CS: cesarean section, NVD: normal vaginal delivery | |||||||||||||
Patient characteristics, diagnosis, management and outcome
The included studies characterized patients with delivery ages ranging from 6.1 to 9.3 months, with a median age of 7.6 months. Most patients were female (72.7%), while 12.1% were male, and in 15.2% of cases, gender was unspecified. Diagnoses were predominantly made during the first and second trimesters (39%) or after birth (33.3%), with cesarean section as the most common delivery mode (66.7%). Tumor weights ranged from 1.5 to 5 kg, with an average diameter of 15.6 cm. Surgical resection was the primary treatment, performed in 93.9% of cases, although two patients died before surgery could be undertaken. Complications were frequent, with respiratory failure as the most common (30.3%). Histopathological analysis indicated that 39.4% of tumors were immature teratomas and 33.3% were mature teratomas. The overall survival rate was 66.7%, and 18.2% of surviving patients experienced tumor recurrence (Table 2).
|
Variables |
Frequency / Percentage |
|
Patient demographics |
|
|
Age at delivery time, range (median, mean ± SD), month |
6.1 – 9.3 (7.6, 7.6 ± 0.97) |
|
Gender Male Female N/A |
4 (12.1%) 24 (72.7%) 5 (15.2%) |
|
Diagnosis time In the first trimester In the second trimester In the third trimester Diagnosed prenatally with unknown trimester Postnatal Unknown |
2 (6.1%) 11 (33.3%) 4 (12.1%) 3 (9.1%) 11 (33.3%) 2 (6.1%) |
|
Mode of delivery Cesarean section Normal vaginal delivery Unknown |
22 (66.7%) 6 (18.2%) 5 (15.1%) |
|
Weight of the tumor (kg), range (mean ± SD) |
1.5 – 5 (3.1 ± 0.96) |
|
Tumor size (cm), range (mean ± SD) |
10 – 30 (15.6 ± 4.77) |
|
Group of tumors (Altman classification) Group 1 Group 2 Group 3 Unknown |
9 (27.3%) 7 (21.2%) 4 (12.1%) 13 (39.4%) |
|
Management Surgical resection Patient died before surgery |
31 (93.9%) 2 (6.1%) |
|
Complications Yes No Unknown |
21 (63.6%) 10 (30.3%) 2 (6.1%) |
|
Common perioperative complications* Respiratory failure Bleeding Cardiac failure/problem Infection |
10 (30.3%) 3 (9.1%) 3 (9.1%) 2 (6.1%) |
|
Histopathology Immature teratoma Mature teratoma Mixed teratoma Unknown |
13 (39.4%) 11 (33.3%) 3 (9.1%) 6 (18.2%) |
|
Survival Yes No Unknown |
22 (66.7%) 5 (15.1%) 6 (18.2%) |
|
Recurrence among survived cases Yes No Unknown |
4 (18.2%) 8 (36.4%) 10 (45.4%) |
|
*Other complications may have been reported N/A: non-available, SD: standard deviation |
|
Complications and recurrence
The comparative analysis of complications assessed the trimester of diagnosis, timing of diagnosis, and recurrence rates. Most complications occurred in the second trimester (10 cases), with fewer cases reported in the first and third trimesters; however, the p-value of 0.34 indicates no significant difference across trimesters. Diagnostic timing was categorized into prenatal and postnatal periods. Complications were more common in the 20 cases diagnosed prenatally (16 cases) than in postnatal diagnoses, but Fisher’s exact test yielded a p-value of 0.10, suggesting no statistically significant association. For recurrence, complication rates were similar in both recurrence and non-recurrence groups, with a p-value of 0.55, supporting the lack of a substantial correlation. Overall findings reveal no strong statistical relationships between complications and diagnostic timing, trimester of diagnosis, or recurrence (Table 3). The influence of tumor size and weight on complications, recurrence, and survival is demonstrated. The mean tumor size was slightly larger in cases with complications (15.02 ± 3.49 cm) compared to those without complications (14.29 ± 3.87 cm), but the difference was not statistically significant (p = 0.6). Recurrence was associated with larger tumors (17.16 ± 7.41 cm) relative to non-recurrent cases (13.42 ± 2.66 cm), although this difference was not significant (p = 0.14). Similarly, non-survivors exhibited a higher mean tumor size (19.25 ± 5.65 cm) compared to survivors (14.6 ± 4.84 cm), but the association was not statistically meaningful (p = 0.41). For tumor weight, no significant variations were found across outcomes. The mean tumor weight in cases with complications was 3.03 ± 0.88 kg versus 3.47 ± 1.09 kg in those without (p = 0.39). Likewise, tumor weight did not show significant differences for recurrence (p = 0.92) or survival (p = 0.35). The findings indicate that neither tumor size nor weight significantly impacted complications, recurrence, or survival (Table 4).
|
Variables |
Complication |
Total |
P-value |
|
|
Yes |
No |
|||
|
Trimester at diagnosis First Second Third |
1 10 3 |
1 1 1 |
2 11 4 |
0.34 |
|
Diagnosis time Prenatal Postnatal |
16 5 |
4 6 |
20 11 |
0.10* |
|
Recurrence Yes |
2 6 |
2 2 |
4 8 |
0.55* |
|
CS; cesarean section, NVD; normal vaginal delivery *Fisher's exact test |
||||
|
Tumor characteristics |
Complication |
P-value |
Recurrence |
P-value |
Survival |
P-value | |||
|
Yes |
No |
Yes |
No |
Yes |
No |
||||
|
Tumor size (mean ±SD) |
15.02 ± 3.49 |
14.29 ± 3.87 |
0.6 |
17.16 ± 7.41 |
13.42 ± 2.66 |
0.14 |
14.6 ± 4.84 |
19.25 ± 5.65 |
0.41 |
|
Tumor weight (mean ±SD) |
3.03 ± 0.88 |
3.47 ± 1.09 |
0.39 |
3.40 ± 1.08 |
3.31 ± 1.04 |
0.92 |
3.25 ± 0.91 |
2.34 ± 0.80 |
0.35 |
| SD; standard deviation | |||||||||
Discussion
SCTs are rare embryonal tumors that develop in the sacrococcygeal region of newborns, the most common site for germ cell tumors. While SCTs occur more frequently in females—with 72.7% of cases in this systematic review involving female patients, they tend to show a higher malignancy rate in males [16]. Most of these tumors are histologically benign and can be classified into three main types: mature teratomas, which consist of fully differentiated tissues such as bone, teeth, and hair; immature teratomas, which contain embryonal elements or partially differentiated structures that pose a significant risk of malignancy; and malignant teratomas, which include one or more malignant germ cell tumors such as yolk sac tumors, choriocarcinomas, and embryonal carcinomas [5,21].
SCTs are classified into four types based on the location of internal and external tumors, as defined by the AAPSS. Type I involves an externally visible mass; Type II is characterized by an external mass with a significant intrapelvic component; Type III includes both external and pelvic masses; and Type IV is entirely internal [17]. Prenatal ultrasound (U/S) can effectively identify Types I and II, while Type IV poses a higher risk of malignancy due to its internal location. Routine U/S is typically performed during the second trimester for antenatal diagnosis, though detection is possible as early as the first trimester. In accordance with this data, more than 60 percent of cases in this systematic review are diagnosed prenatally. However, many pregnant women skip first-trimester U/S screenings, making early detection of masses more challenging. In some cases, the mass is large enough to be detected earlier [1].
Magnetic resonance (MR) imaging complements ultrasound by providing a more detailed characterization of the mass’s tissue components [16]. It is particularly useful for evaluating the full extent of large lesions. MR imaging can accurately determine the tumor’s reach and the pressure it exerts on surrounding organs. It also aids in distinguishing this condition from common differential diagnoses, such as distal neural tube defects, including myelocystocele or myelomeningocele [5].
Additionally, elevated levels of tumor markers, such as alpha-fetoprotein (AFP), are used to assess the likelihood of malignancy. High AFP levels may indicate a malignant tumor; however, in newborns, AFP levels are naturally elevated and typically return to normal by around nine months of age, leading to potential misdiagnosis of the condition as malignant [1].
Perinatal morbidity and mortality rates are notably elevated in fetuses with SCT, primarily due to the high frequency of preterm birth. The most severe perinatal complications include premature labor, malignant tumor infiltration, hemorrhage or rupture of the tumor, amniotic fluid obstruction, and heart failure [5]. Factors that increase the risk of complications include rapid tumor growth exceeding 150 cm³ per week, tumor size greater than 10 cm, highly vascularized solid tumors, polyhydramnios, and cardiac decompensation [4,21].
Cesarean delivery is recommended for mothers carrying fetuses with SCTs larger than 10 cm in diameter, especially when the tumors are highly vascularized or exceed 5 cm. This approach minimizes the risk of tumor rupture and hemorrhage [2]. This systematic review indicates that cesarean section is performed in more than two-thirds of such cases. The incision is typically made in the lower uterine segment, as the uterus often becomes significantly enlarged due to the tumor's size [21].
Timely surgical intervention for SCTs is crucial, particularly when the tumor exceeds 10 cm, as delays can increase the risk of malignancy and recurrence. Surgery is the primary treatment, with over 93% of cases requiring surgical management. Current expert recommendations indicate that coccygectomy is the most effective approach for preventing the recurrence of benign teratomas, with minimal risk to the tumor cyst wall [2,4]. Ideally, this procedure should be performed within one week and not delayed beyond that period. During surgery, careful fetal monitoring is essential to prevent operative mortality, often caused by severe hemorrhage or cardiac arrest, frequently linked to hyperkalemia [4,10]. Additionally, ensuring the availability of cross-matched blood in the operating room is critical [4].
When the coccyx is not removed, the likelihood of cancer recurrence can be as high as 37%. Even when patients have the entire coccyx removed, there is still a chance of the cancer returning, ranging from 11% to 22% [2]. This aligns with the 18% recurrence rate mentioned in this review. Although recurrence is uncommon, several factors could contribute to it, such as incomplete removal of the tumor, failure to completely remove the coccyx along with the tumor, tumor spillage or rupture, and undetected malignant components within the tumor [12].
Although survival outcomes for SCTs are generally positive, the mortality rate for tumors larger than 10 cm is reported to be around 18%, which is nearly similar to the 15% rate mentioned in this review. SCTs can potentially recur years after treatment, highlighting the importance of continuous monitoring into adulthood. Regular follow-up appointments are recommended every 3 to 6 months, including physical examinations, such as rectal exams, diagnostic imaging, and alpha-fetoprotein (AFP) testing, for at least three years [1,16].
Conclusion
Routine ultrasound and magnetic resonance imaging play critical roles in the antenatal diagnosis and characterization of SCTs. Given the elevated perinatal morbidity and mortality associated with SCTs, especially due to preterm birth, cesarean delivery is advised for tumors larger than 10 cm to reduce risks of rupture and bleeding. Furthermore, coccygectomy is highlighted as the most effective strategy for preventing the recurrence of benign teratomas, underscoring the importance of timely and thorough intervention in managing these cases.
Declarations
Conflicts of interest: The author(s) have no conflicts of interest to disclose.
Ethical approval: Not applicable, as systematic reviews do not require ethical approval.
Patient consent (participation and publication): Not applicable.
Funding: The present study received no financial support.
Acknowledgements: None to be declared.
Authors' contributions: PAN, and BAA were responsible for the manuscript's data collection, analysis, and final approval. GSS, WNS and SI were significant contributors to the conception of the study, as well as to the literature search for related studies. BAM, SFA, and SMA were involved in the literature review, the design of the study, and the critical revision of the manuscript. SMA and PAN were involved in the literature review, the writing of the manuscript, and the design of the study and interpretation. WNS and SMA confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Use of AI: AI was not used in the drafting of the manuscript, the production of graphical elements, or the collection and analysis of data.
Data availability statement: Note applicable.
References
- Sabir WN, Ahmed SM, Hasan KM, Mohammed BA, Kareem HO, Najmadden ZB, et al. Giant sacrococcygeal teratoma in an infant: a case report with a literature review. Annals of Medicine and Surgery. 2023 Nov 1;85(11):5666-9. doi:10.1097/MS9.0000000000001274
- Meshram RM, Nikila R, Nagdive N. Giant sacrococcygeal teratoma in neonate: A case report and review of literature. Indian Journal of Medical and Paediatric Oncology. 2019 Oct;40(04):573-5. doi:10.4103/ijmpo.ijmpo_16_18
- Roka YB, Shrestha M, Pandey SR. Sacrococcygeal Teratoma in a Child: A Case Report. Journal of Nepal Paediatric Society. 2010 Jul 1;30(2). doi:10.3126/jnps.v30i2.3450
- Boumediene AB, KACIMI SE, RIFFI O, BOGHARI C, DJEDID SN. Mature ruptured sacrococcygeal teratoma in a 1-day old neonate. Journal of Pediatric Surgery Case Reports. 2022 Jun 1;81:102245. doi:10.1016/j.epsc.2022.102245
- Neupane D, Dahal A, Lageju N, Jaiswal LS, Bista N, Sapkota A. Giant sacrococcygeal teratoma in a neonate: illustrative case. Journal of Neurosurgery: Case Lessons. 2022 May 9;3(19). doi:10.3171/CASE22125
- Mohammed, K. K., Hama, J. I., Kakamad, F. H. Beyond Beall's list: The need for contemporary evaluation tools in predatory publishing research. Learned Publishing. 2024;37(2), 157-158 doi:10.1002/leap.1602
- Chamberlin E, Lewis M, Fuentes K, Sullivan K. Near Cardiac Arrest in a Neonate with Massive Sacrococcygeal Teratoma. doi:N/A
- Dey A, Wyrebek R, Torres L, Escoto D, Shakeel F, Mayer J. Tumor lysis syndrome in premature infant prompting early resection of a large sacrococcygeal teratoma: a case report. BMC pediatrics. 2023 Sep 2;23(1):440. doi:10.1186/s12887-023-04193-w
- Koç Y, Doğan Ö, Özmert S. Anesthesia Management of the Premature Newborn with Giant Sacrococcygeal Teratoma. Turkish Journal of Pediatric Disease. 2022 Nov 1;16(6):555-8. doi:10.12956/tchd.1107639
- Zvizdic Z, Rustempasic N, Pasic IS, Bilalovic N, Chikha A, Mesic A, et al. A large, highly vascularized sacrococcygeal teratoma in a preterm male infant: A case report. doi:10.1016/j.asjsur.2020.12.027
- Guitart J, Teixidor M, Brun N, López S, Criado E, Romero N. Preoperative giant sacrococcygeal teratoma embolization in a newborn—A case report and a review. Cir Pediatr. 2020 Apr 1;33(2):95-8. doi:N/A
- Savitri QM, Prihartono S, Harahap A. Giant sacrococcygeal teratoma in newborn: A rare case. Journal of Pediatric Surgery Case Reports. 2019 Aug 1;47:101223. doi:10.1016/j.epsc.2019.101223
- Singhal N, Gupta N, Kulhari S. Fetiform Sacrococcygeal Teratoma-In A Newborn A Case Report. doi:10.9790/0853-1708101416
- Konoplitskyi VS, Pogorily VV, Dubrovin AG, Moravska OA, Chuhu TV, Yakymenko AG, et al. Strategy and medical approach to treatment of giant sacrococcygeal teratoblastoma with concomitant teratoma in a newborn child. PAEDIATRIC SURGERY. UKRAINE. 2018(1 (58)):61-5. doi:10.15574/PS.2018.58.61
- Lee BS. A Contrast Nephropathy in a Preterm Infant Following Preoperative Embolization of Giant Sacrococcygeal Teratoma. Childhood Kidney Diseases. 2017;21(1):26-30. doi:10.3339/jkspn.2017.21.1.26
- Bechtel AS, Gauger CA. Sacrococcygeal teratoma with multiple recurrences by intradural extension in the neonate: A common neonatal mass with an unusual course. Global Pediatric Health. 2014 Nov 25;1:2333794X14560820. doi:10.1177/2333794X14560820
- Mondal M, Biswas B, Roy A, Ari A, Nayek K, Datta AK, et al. A neglected case of Sacrococcygeal teratoma in a neonate. Asian Journal of Medical Sciences. 2015;6(2):108-10. doi:10.3126/ajms.v6i2.10448
- Mbumba B, Massez A, Lingier P, Donner C, Vermeylen D, Makulo JR, et al. Antenatal diagnosis of a sacrococcygeal teratoma. Journal of the Belgian Society of Radiology. 2010 Jun 10;93(6):314-6. doi:10.5334/jbr-btr.350
- Lahdes-Vasama TT, Korhonen PH, Seppänen JM, Tammela OK, Iber T. Preoperative embolization of giant sacrococcygeal teratoma in a premature newborn. Journal of Pediatric Surgery. 2011 Jan 1;46(1):e5-8. doi:10.1016/j.jpedsurg.2010.09.038
- Abraham E, Parray T, Ghafoor A. Complications with massive sacrococcygeal tumor resection on a premature neonate. Journal of anesthesia. 2010 Dec;24:951-4. doi:10.1007/s00540-010-1027-x
- Den Otter SC, De Mol AC, Eggink AJ, Van Heijst AF, De Bruijn D, Wijnen RM. Major sacrococcygeal teratoma in an extreme premature infant: a multidisciplinary approach. Fetal diagnosis and therapy. 2007 Oct 9;23(1):41-5. doi:10.1159/000109225
- Howman-Giles R, Holland AJ, Mihm D, Montfort JM, Arbuckle S, Kellie S. Somatic malignant transformation in a sacrococcygeal teratoma in a child and the use of F 18 FDG PET imaging. Pediatric surgery international. 2008 Apr;24:475-8. doi:10.1007/s00383-007-2006-7
- Hosono S, Mugishima H, Nakano Y, Murabayashi M, Shimada M, Minato M, et al. Autologous cord blood transfusion in an infant with a huge sacrococcygeal teratoma. doi:10.1515/JPM.2004.035
- Ribeiro PR, Guys JM, Lena G. Sacrococcygeal teratoma with an intradural and extramedullary extension in a neonate: case report. Neurosurgery. 1999 Feb 1;44(2):398-400. doi:N/A
- Jona JZ. Progressive tumor necrosis and lethal hyperkalemia in a neonate with sacrococcygeal teratoma (SCT). Journal of Perinatology. 1999 Oct;19(7):538-40. doi:10.1038/sj.jp.7200197
- Johnston PW. The diagnostic value of alpha-fetoprotein in an infant with sacrococcygeal teratoma. Journal of pediatric surgery. 1988 Sep 1;23(9):862-3. doi:10.1016/S0022-3468(88)80244-6
- Robertson FM, Crombleholme TM, Frantz III ID, Shephard BA, Bianchi DW, D'Alton ME. Devascularization and staged resection of giant sacrococcygeal teratoma in the premature infant. Journal of pediatric surgery. 1995 Feb 1;30(2):309-11. doi:10.1016/0022-3468(95)90579-0
- Inoue M, Kubota A, Hasegawa T, Hata S, Takahashi E, Kawahara H, et al. Antenatal diagnosis of sacrococcygeal teratoma with hydrops fetalis; a case report. European journal of pediatric surgery. 1994 Apr;4(02):125-7. doi:10.1055/s-2008-1066085
- Nakayama DK, Killian A, Hill LM, Miller JP, Hannakan C, Lloyd DA, et al. The newborn with hydrops and sacrococcygeal teratoma. Journal of pediatric surgery. 1991 Dec 1;26(12):1435-8. doi:10.1016/0022-3468(91)91060-C
- Worsham GF, Beckman EN, Mitchell EH. Sacrococcygeal teratoma in a neonate: association with maternal use of acetazolamide. JAMA. 1978 Jul 21;240(3):251-2. doi:10.1001/jama.1978.03290030069029
- Williams AO, Lagundoye SB, Bankole MA. Sacrococcygeal teratoma in Nigerian children. Archives of Disease in Childhood. 1970 Feb 1;45(239):110-3. doi:10.1136/adc.45.239.110
- Schiffer MA, Geenberg E. Sacrococcygeal teratoma in labor and the newborn. American Journal of Obstetrics and Gynecology. 1956 Nov 1;72(5):1054-62. doi:10.1016/0002-9378(56)90071-0
- WALKER JM, FOSTER RJ. Sacrococcygeal teratoma in the newborn. AMA Archives of Surgery. 1950 Dec 1;61(6):1138-44. doi:10.1001/archsurg.1950.01250021148013

This work is licensed under a Creative Commons Attribution 4.0 International License.

